Abstract
BACKGROUND: Obesity may increase the risk of respiratory failure after cardiothoracic surgery. A recruitment maneuver followed by PEEP might decrease the risk of respiratory failure in obese subjects. We hypothesized that the routine use after heart surgery of a recruitment maneuver followed by high or low PEEP level would decrease the frequency of respiratory failure in obese subjects.
METHODS: In a pragmatic, randomized controlled trial, we assigned obese subjects (ie, with body mass index [BMI] ≥ 30 kg/m2) in the immediate postoperative period of cardiothoracic surgery to either volume control ventilation with 5 cm H2O of PEEP (control group) or a recruitment maneuver followed by 5 or 10 cm H2O of PEEP in the intervention arms (RM5 and RM10 groups, respectively). The primary outcome was the proportion of subjects with postextubation respiratory failure, defined as the need for re-intubation, bi-level positive airway pressure, or high-flow nasal cannula within the first 48 h.
RESULTS: The study included 192 subjects: 65 in the control group (BMI 33.5 ± 3.2 kg/m2), 66 in the RM5 group (BMI 34.5 ± 3.2 kg/m2, and 61 in RM10 group (BMI 33.8 ± 4.8 kg/m2). Postextubation respiratory failure occurred in 14 subjects in the control group (21.5% [95% CI 13.3–35.3]), 21 subjects in the RM5 group (31.8% [95% CI 21.2–44.6]), and 9 subjects in the RM10 group (14.7% [95% CI 7.4–26.7]) (P = .07). The recruitment maneuver was stopped prematurely due to severe hypotension in 8 (12.1%) RM5 subjects and in 4 (6.6%) RM10 subjects (P = .28). There were no significant differences between the 3 groups for the frequencies of atelectasis, pneumonia, and death in the ICU.
CONCLUSIONS: The routine use after heart surgery of a recruitment maneuver followed by 5 or 10 cm H2O of PEEP did not decrease the frequency of respiratory failure in obese subjects. A recruitment maneuver followed by 5 cm H2O of PEEP is inappropriate.
- obesity
- cardiac surgery
- recruitment maneuver
- positive end-expiratory pressure
- respiratory failure
- randomized controlled trial
Introduction
Obesity, defined as a body mass index ≥ 30 kg/m2, adversely affects respiratory function by decreasing respiratory system compliance, increasing pulmonary resistance, and diminishing functional residual capacity and alveolar oxygenation.1 Atelectasis is a major concern after surgery in obese subjects.2 Atelectasis increases intrapulmonary shunting, thereby inducing hypoxemia3 while also promoting bacterial growth.2 On-pump heart surgery has been reported to produce immediate but transient impairments in lung and chest wall mechanics.4 Severe hypoxemia is common after heart surgery,5,6 and obesity increases the risk of postoperative hypoxemia.6,7 These data suggest that obesity may increase the risk of respiratory failure after heart surgery.
To decrease the risk of postoperative respiratory failure, several strategies have been proposed, including a recruitment maneuver (RM) and PEEP. RM and PEEP have been applied alone or in combination with different levels of PEEP. The goal of RM is to open collapsed alveoli, after which PEEP is applied to keep the lungs open.2,8,9 However, adverse hemodynamic effects must be taken into account, especially after cardiac surgery.10 A nationwide survey in France reported that perioperative applications of RM and PEEP are unusual.11
Two randomized controlled trial (RCT) have been performed in nonobese subjects in the perioperative period of cardiac surgery with differing results. Intraoperative RM and PEEP failed to decrease pulmonary complications after elective on-pump heart surgery.12 In the immediate postoperative period, hypoxemic subjects who were randomized to intensive RM had fewer pulmonary complications compared to those in the moderate RM arm.13
We hypothesized that the routine use after heart surgery of a RM followed by PEEP would decrease the frequency of respiratory failure in obese subjects. Considering the possible deleterious effects of PEEP and that the optimal level has not been determined, we tested in a pragmatic RCT whether postoperative RM followed by high (10 cm H2O) or low (5 cm H2O) PEEP decreased the frequency of postextubation respiratory failure in obese subjects after heart surgery.
Quick Look
Current Knowledge
The goal of recruitment maneuvers is to open collapsed alveoli, after which PEEP is applied to keep the lungs open. In the immediate postoperative period of cardiac surgery, hypoxemic nonobese subjects who were randomized to intensive recruitment maneuver had fewer pulmonary complications compared to those in the moderate recruitment maneuver arm. Clinical studies have not confirmed these findings in obese subjects.
What This Paper Contributes to Our Knowledge
In a randomized controlled trial, the routine use of recruitment maneuver after cardiothoracic surgery followed by 5 or 10 cm H2O of PEEP did not decrease the frequency of respiratory failure in obese subjects.
Methods
Subjects and Study Design
Subjects were eligible if they had undergone cardiac and thoracic aortic procedures and had a body mass index ≥ 30 kg/m2. Exclusion criteria were age < 18 y or > 80 y, obstructive sleep apnea syndrome treated with CPAP, left-ventricular ejection fraction < 25%, hemodynamic instability, need for emergent extracorporeal membrane oxygenation, use of intraaortic balloon counterpulsation, pregnancy, enrollment in another study, and being ineligible for coverage by the French statutory health care insurance system.
A pragmatic RCT in 3 parallel groups of subjects was conducted in 2 ICUs in France. The study protocol was approved by the CPP Ile-de-France VII (December 10, 2014, IRCB #2014-A01558-39). Written and oral information was provided to the subjects or relatives. A non-opposition consent was obtained according to the CPP’s request. The trial was registered on the Australian New Zealand Clinical Trials Registry (#ACTRN12616000155493).
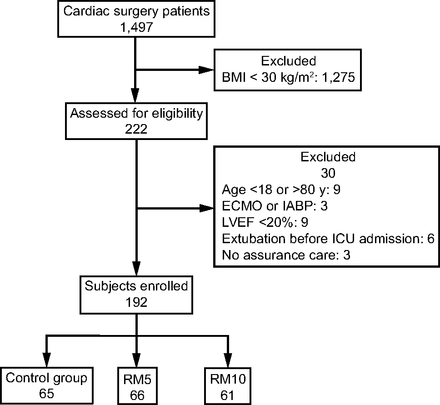
Permuted block randomization in a 1:1:1 ratio was performed using a single, computer-generated, random-number sequence for both centers (nQuery Advisor, Statsols, Cork, Ireland). Concealment was with opaque envelopes. Senior ICU physicians enrolled and randomized the subjects (Fig. 1). Blinding was not feasible.
Flow chart. BMI = body mass index; ECMO = extra corporeal membrane oxygenation; IABP = intra-aortic balloon counterpulsation; LVEF = left-ventricular ejection fraction; RM = recruitment maneuver.
Study Interventions
The anesthetic management and postoperative care are detailed in the supplemental material (available at http://www.rcjournal.com). In the control group, volume control ventilation was continued in the ICU after surgery, with 5 cm H2O of PEEP and 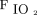 set to 0.7. Thereafter,
set to 0.7. Thereafter,  was set to achieve > 92%
was set to achieve > 92% 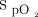 until weaning criteria were met. RMs were conducted in the ICU when the chest radiograph ruled out a pneumothorax and after the subject received 1,000 mL crystalloids. In the RM5 group, an RM was performed, followed by the same ventilation strategy as in the control group. The intervention in the RM10 group differed from that in the RM5 group only regarding the PEEP level, which was set at 10 cm H2O. The RM in both intervention groups was conducted using pressure control ventilation with 15 cm H2O of PEEP, 30 cm H2O of driving pressure, a breathing frequency of 20 breaths/min, and an inspiratory time of 1.5 s for 4 min.14 The RM was performed only in subjects without pneumothorax and with a compatible hemodynamic state (defined as mean arterial pressure > 65 mm Hg and norepinephrine < 0.5 µg/kg/min). The RM was stopped if the mean arterial pressure fell to < 40 mm Hg or the systolic blood pressure decreased by > 20% with a mean arterial pressure < 65 mm Hg or
until weaning criteria were met. RMs were conducted in the ICU when the chest radiograph ruled out a pneumothorax and after the subject received 1,000 mL crystalloids. In the RM5 group, an RM was performed, followed by the same ventilation strategy as in the control group. The intervention in the RM10 group differed from that in the RM5 group only regarding the PEEP level, which was set at 10 cm H2O. The RM in both intervention groups was conducted using pressure control ventilation with 15 cm H2O of PEEP, 30 cm H2O of driving pressure, a breathing frequency of 20 breaths/min, and an inspiratory time of 1.5 s for 4 min.14 The RM was performed only in subjects without pneumothorax and with a compatible hemodynamic state (defined as mean arterial pressure > 65 mm Hg and norepinephrine < 0.5 µg/kg/min). The RM was stopped if the mean arterial pressure fell to < 40 mm Hg or the systolic blood pressure decreased by > 20% with a mean arterial pressure < 65 mm Hg or 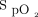 < 85%.
< 85%.
The weaning procedure was the same in all 3 groups. When weaning criteria were met,15 a spontaneous breathing trial with a T-tube was performed for 30–120 min according to the standardized protocol used in each ICU. Oxygen was provided (maximum 9 L/min) to obtain  ≥ 92%. After extubation, supplemental oxygen up to 6 L/min was given routinely to maintain
≥ 92%. After extubation, supplemental oxygen up to 6 L/min was given routinely to maintain 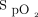 > 92%. All subjects followed an active physical therapy program and received chest physiotherapy twice between 7:00 am and 7:00 pm, or more often if needed. The other components of postoperative care were at the discretion of the attending physicians, who followed local protocols for analgesia, blood transfusions, albumin therapy, and medical treatments for bleeding.
> 92%. All subjects followed an active physical therapy program and received chest physiotherapy twice between 7:00 am and 7:00 pm, or more often if needed. The other components of postoperative care were at the discretion of the attending physicians, who followed local protocols for analgesia, blood transfusions, albumin therapy, and medical treatments for bleeding.
Data Collection
We recorded the following data: age, sex, weight, height, current smoking, Simplified Acute Physiology Score (SAPS) II used as an index of acute illness severity, left-ventricular ejection fraction, types of surgical procedures, emergency procedures, use of cardiopulmonary bypass and duration, volume of intraoperative fluids infused, use of intraoperative drugs, use of blood products, duration of mechanical ventilation, length of ICU and hospital stay, and ICU and 28-d mortality.
Study Outcomes and Definitions
The primary outcome was postextubation respiratory failure, which was defined as the use of invasive mechanical ventilation or noninvasive ventilation provided as bi-level positive airway pressure (BPAP), or high-flow nasal cannula (HFNC) within 48 h after extubation. The same composite outcome was used previously.16 Criteria for starting BPAP or HFNC were previously reported16:  < 300 and/or breathing frequency > 25 breaths/min for at least 2 h and/or accessory respiratory muscle activation and/or paradoxical respiration. The choice between BPAP and HFNC was at the discretion of the attending physicians, despite evidence of better
< 300 and/or breathing frequency > 25 breaths/min for at least 2 h and/or accessory respiratory muscle activation and/or paradoxical respiration. The choice between BPAP and HFNC was at the discretion of the attending physicians, despite evidence of better 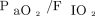 values with BPAP.17 Re-intubation decisions were made by the attending physicians on the basis of criteria previously reported by our team16 (ie, respiratory arrest, apneas with loss of consciousness, gasping, encephalopathy, hemodynamic instability, unmanageable secretions, clinical signs of exhaustion, refractory hypoxemia (
values with BPAP.17 Re-intubation decisions were made by the attending physicians on the basis of criteria previously reported by our team16 (ie, respiratory arrest, apneas with loss of consciousness, gasping, encephalopathy, hemodynamic instability, unmanageable secretions, clinical signs of exhaustion, refractory hypoxemia (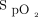 < 88% with
< 88% with 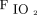 = 1.0), or respiratory acidosis (pH < 7.30 and partial pressure of arterial CO2 ≥ 50 mm Hg).
= 1.0), or respiratory acidosis (pH < 7.30 and partial pressure of arterial CO2 ≥ 50 mm Hg).
The secondary outcome measures were 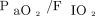 and breathing frequency changes after the spontaneous breathing trial and between 6 and 12 h after extubation; the frequencies of postoperative pneumonia, pneumothorax requiring drainage, or atelectasis; the time course of the Sequential Organ Failure Assessment (SOFA) score;16 duration of invasive mechanical ventilation; length of ICU and hospital stay; and mortality in the ICU and within 28 d after surgery. We also studied the proportion of subjects without postextubation respiratory failure within the 7 d after extubation according to treatment arm.
and breathing frequency changes after the spontaneous breathing trial and between 6 and 12 h after extubation; the frequencies of postoperative pneumonia, pneumothorax requiring drainage, or atelectasis; the time course of the Sequential Organ Failure Assessment (SOFA) score;16 duration of invasive mechanical ventilation; length of ICU and hospital stay; and mortality in the ICU and within 28 d after surgery. We also studied the proportion of subjects without postextubation respiratory failure within the 7 d after extubation according to treatment arm.
Nosocomial pneumonia was defined as a clinical suspicion confirmed with positive bacteriological cultures (broncho alveolar lavage ≥104 cfu/ml, endotracheal aspirate ≥105 cfu/ml, spontaneous sputum ≥107 cfu/ml) from deep lung specimens. Cases of nosocomial pneumonia were recorded throughout the ICU stay.16 The diagnosis of atelectasis was based on a new lung opacity on a chest radiograph with a shift in the mediastinum or ipsilateral hemi-diaphragm.12
Statistical Analysis
Based on the ARISCAT score,18 we expected postoperative pulmonary complications to occur in 42% of subjects. To obtain 80% power for detecting at least a 20% decrease in the frequency of postextubation respiratory failure with the 2-sided alpha risk set at 5%, 192 subjects (64 in each group) were required.
All analyses were performed on an intention-to-treat basis. Dichotomous variables were compared using the chi-square test as appropriate. Categorical variables were described as number (frequency) with 95% CI. Continuous variables were described as the mean ± SD and compared using analysis of variance (ANOVA) when normally distributed; if not, they were described as the median (interquartile range) and compared using the Kruskal-Wallis test. When ANOVA or Kruskal-Wallis tests showed differences, comparisons between groups were performed using the t test or the Mann-Whitney U test, as appropriate. Changes in quantitative repeatedly measured variables within the 3 groups were assessed using ANOVA followed by the Scheffe test. A post hoc analysis was performed in subgroups defined by a baseline 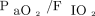 < 200 or ≥ 200 mm Hg for the primary outcome. Kaplan-Meier plots of postextubation respiratory failure during the first 7 d after extubation were compared by applying the log-rank test. P values < .05 were considered statistically significant.
< 200 or ≥ 200 mm Hg for the primary outcome. Kaplan-Meier plots of postextubation respiratory failure during the first 7 d after extubation were compared by applying the log-rank test. P values < .05 were considered statistically significant.
Risk factors for postextubation respiratory failure were sought by multivariate logistic regression. The odds ratios (ORs) with their 95% CIs were computed. The multivariate model used variables associated with P values ≤ .10 in the univariate analyses of risk factors for postextubation respiratory failure, as well as 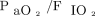 < 200 mm Hg before RM. The R2 was computed to assess model performance.
< 200 mm Hg before RM. The R2 was computed to assess model performance.
Results
Subjects
Figure 1 is the study flow chart. Of the 1,497 patients screened for eligibility between February 11, 2016, and December 22, 2017, 222 (19.0%) were obese. Among them, 192 were randomized: 65 to the control group, 66 to the RM5 group, and 61 to the RM10 group; 162 subjects were at Marie Lannelongue Hospital and 30 subjects were at la Clinique de la Sauvegarde. All 192 subjects completed the study. Table 1 lists their baseline characteristics, which were similar across the 3 groups, except for smaller proportions of subjects requiring blood products and mitral valve surgery in the control group.
Baseline Subject Characteristics
Primary Outcome
The proportion of subjects with postextubation respiratory failure within the first 48 h postextubation was 21.5% (95% CI 13–35) in the control group, 31.8% (95% CI 21–45) in the RM5 group, and 14.7% (95% CI 7–27) in the RM10 group (P = .07) (Table 2). Extubation was performed on the day of surgery (day 0) in 86% of controls, 73% of RM5 subjects, and 80% of RM10 subjects (P = .16). Time from ICU admission to extubation on day 0 was 5.0 ± 2.4, 4.7 ± 1.3, and 5.1 ± 2.5 h in the control, RM5, and RM10 groups, respectively (P = .70).
Subject Outcomes
In the subjects with postextubation respiratory failure, the median (interquartile range [IQR]) time to first use of BPAP, HFNC, or invasive mechanical ventilation was similar in the 3 groups: 1.0 d (IQR 0.5–1.0), 1.0 d (IQR 0.5–1.0), and 1.0 d (IQR 0.5–1.0) in the control, RM5, and RM10 groups, respectively (P = .82). The use of HFNC was similar among the 3 groups at 86%, 80%, and 55% in the control, RM5, and RM10 groups, respectively (P = .22). BPAP and HFNC duration was 2.9 ± 1.9 d, 1.9 ± 1.1 d, and 2.2 ± 1.6 d in the control, RM5, and RM10 groups, respectively (P = .23).
The post hoc analysis demonstrated a significant interaction between 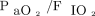 just before the RM (< 200 mm Hg vs ≥ 200 mm Hg) and the treatment group regarding postextubation respiratory failure (P = .030). In the subgroups with a pre-RM
just before the RM (< 200 mm Hg vs ≥ 200 mm Hg) and the treatment group regarding postextubation respiratory failure (P = .030). In the subgroups with a pre-RM 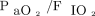 ≥ 200 mm Hg, the proportion of subjects with postextubation respiratory failure in the RM5 group was significantly higher than in the RM10 group (P = .01) but similar to that in the control group (P = .14) (see the supplementary materials at http://www.rcjournal.com).
≥ 200 mm Hg, the proportion of subjects with postextubation respiratory failure in the RM5 group was significantly higher than in the RM10 group (P = .01) but similar to that in the control group (P = .14) (see the supplementary materials at http://www.rcjournal.com).
The factors entered into the multivariate logistic regression analysis were treatment arm, mitral valve surgery,  < 200 mm Hg before RM, on-pump time, and blood product administration. Two independent risk factors were identified:
< 200 mm Hg before RM, on-pump time, and blood product administration. Two independent risk factors were identified: 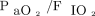 < 200 mm Hg before RM (odds ratio 3.13 [95% CI 1.50–6.51], P = .002) and longer on-pump time (odds ratio 1.18 per additional 15 min [95% CI 1.02–1.36, P = .02). Neither treatment arm (RM5 and RM10 groups) was identified as a risk factor or protective for postoperative respiratory failure in this cohort. The R2 value was 0.12.
< 200 mm Hg before RM (odds ratio 3.13 [95% CI 1.50–6.51], P = .002) and longer on-pump time (odds ratio 1.18 per additional 15 min [95% CI 1.02–1.36, P = .02). Neither treatment arm (RM5 and RM10 groups) was identified as a risk factor or protective for postoperative respiratory failure in this cohort. The R2 value was 0.12.
Secondary Outcomes
Within 2 min of the start of the RM, we observed a decrease in systolic, diastolic, and mean arterial pressure. After ceasing the RM, mean arterial pressure recovered without significant differences between groups. The RM was stopped prematurely due to severe hypotension in 8 subjects (12.1% [95% CI 6.3–22.1]) in the RM5 group and 4 subjects (6.6% [95% CI 2.6–15.7]) in the RM10 group (P = .28). Changes in hemodynamic variables before, during, and after the RM are reported in the supplementary materials (available at http://www.rcjournal.com). No subject experienced desaturation ( < 85%) during RM. Following RM, 1 subject in the RM5 group suffered from pneumothorax. The intravenous fluid volume administered during the first 24 h in the ICU was similar across the 3 groups (1,985 ± 1,066, 2,123 ± 833, and 2,240 ± 762 mL in the control, RM5, and RM10 groups, respectively (P = .36).
< 85%) during RM. Following RM, 1 subject in the RM5 group suffered from pneumothorax. The intravenous fluid volume administered during the first 24 h in the ICU was similar across the 3 groups (1,985 ± 1,066, 2,123 ± 833, and 2,240 ± 762 mL in the control, RM5, and RM10 groups, respectively (P = .36).
At baseline, neither respiratory variables nor blood gas levels showed any significant differences across the 3 groups (Table 3). The RM was followed by a 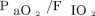 increase, whose magnitude differed between the 2 RM groups (P = .044, repeated measures ANOVA). However, the percentage of
increase, whose magnitude differed between the 2 RM groups (P = .044, repeated measures ANOVA). However, the percentage of 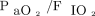 increase after the RM was not significantly different between the RM5 and RM10 groups (36% (55) vs 55% (60), respectively, P = .09). There were no significant differences in
increase after the RM was not significantly different between the RM5 and RM10 groups (36% (55) vs 55% (60), respectively, P = .09). There were no significant differences in  values across the 3 groups after the spontaneous breathing trial (P = .16) (Table 3).
values across the 3 groups after the spontaneous breathing trial (P = .16) (Table 3).
Respiratory Variables and Arterial Blood Gas Values
Table 4 reports respiratory complications and deaths. None of the variables differed significantly across the 3 groups. The proportion of subjects without postextubation respiratory failure within 7 d after extubation, according to treatment arm, are depicted in Figure 2. There were no statistically significant differences between the 3 groups (P = .08 log-rank test).
Absence of postextubation respiratory failure within 7 d after extubation in obese subjects who underwent heart surgery and randomized to a recruitment maneuver (RM) followed by 5 or 10 cm H2O PEEP (RM5 and RM10 groups, respectively) or with no RM and with 5 cm H2O PEEP (control).
Secondary Outcomes
Discussion
Application of a RM followed by a PEEP level of 5 or 10 cm H2O did not reduce postextubation respiratory failure in the first 48 h following extubation of obese subjects after cardiac surgery. Moreover, a RM followed by 5 cm H2O was associated with adverse events. Hypotension was the main complication of RM, but < 10% of subjects required premature RM discontinuation. Neither the frequency of atelectasis nor the length of the hospital stays differed across treatment arms.
Obesity produces changes to lung mechanics that can be immediately exacerbated by surgery and cardiopulmonary bypass, leading to atelectasis. However, at 4 h after surgery, all mechanics returned to normal.4 Atelectasis occurred postoperatively in 17–25% of our obese subjects, which is close to the previously reported frequency of 12–27% in nonobese subjects.12,19 Atelectasis has been reported to last longer in obese subjects.3 Whether theoretical considerations suggest that RMs and PEEP might improve postoperative outcomes in obese patients,2,8,20 the level of evidence is low. We are not aware of any previous RCTs focusing specifically on cardiac surgery subjects with obesity. RM increased end-expiratory lung volume and improved oxygenation,21 and high PEEP increased lung volume and ventilation distribution uniformity while decreasing pendelluft and the frequency of atelectasis.22
In our study, the postoperative application of an RM improved 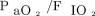 , which is in agreement with previous studies in nonobese subjects.23-25 However, this effect is transient and oxygenation levels were similar among the 3 groups during a spontaneous breathing trial and at 6–12 h after extubation, confirming results of several studies.23,24 There was no significant nor sustainable change in end-expiratory volume when combining a RM with PEEP.24,26 However, in 2 small RCTs, open lung ventilation applied after heart surgery decreased the occurrence of postoperative hypoxemia.26,27
, which is in agreement with previous studies in nonobese subjects.23-25 However, this effect is transient and oxygenation levels were similar among the 3 groups during a spontaneous breathing trial and at 6–12 h after extubation, confirming results of several studies.23,24 There was no significant nor sustainable change in end-expiratory volume when combining a RM with PEEP.24,26 However, in 2 small RCTs, open lung ventilation applied after heart surgery decreased the occurrence of postoperative hypoxemia.26,27
In a large RCT involving nonobese subjects after cardiac surgery, an intensive alveolar recruitment strategy (ie, 3 inflation cycles of 60 s with PEEP set at 30 cm H2O and a driving pressure of 15 cm H2O, followed by PEEP set at 13 cm H2O) decreased the occurrence of severe pulmonary complications up to the fifth postoperative day compared to a moderate recruitment strategy (ie, 3 inflation cycles of 30 s under CPAP set at 20 cm H2O, followed by PEEP set at 8 cm H2O).13 Interestingly, the number of subjects requiring noninvasive ventilation was lower in the intensive recruitment strategy group (4% vs 15%).13 Several differences in the design of this study compared to ours must be mentioned. The level of PEEP was much higher during RM, which was repeated twice before weaning. The duration of mechanical ventilation using a PEEP of 13 cm H2O was 4 h before the weaning process,13 which can decrease the dynamic instabilities in the inflating lung.28 Finally, the amount of postoperative fluid administration was not reported, and we can speculate that higher PEEP level may have reduced pulmonary capillary leak and left-ventricular afterload in case of fluid overload.29
RM followed by a PEEP level of 5 cm H2O seems not appropriate, particularly in less hypoxemic subjects. We used a low tidal volume in the intraoperative and postoperative periods of cardiac surgery,30 which could promote alveolar collapse or atelectasis in dependent lung zones.8,31 A possible alveolar de-recruitment associated with low tidal volume can be recovered by increasing PEEP.32 We hypothesized that alveoli opened after RM but may collapse, and many recruited alveoli that do not collapse are unstable28 and are therefore susceptible to recruitment/de-recruitment-induced injury because a PEEP level of 5 cm H2O is probably inadequate.33
Another matter of concern is the time of application of a RM in the preoperative or postoperative period. At the end of cardiopulmonary bypass, all our subjects have had a RM not followed by PEEP. In a recent RCT involving nonobese cardiac surgery subjects, RM and PEEP applied intraoperatively with maintained ventilation during cardiopulmonary bypass did not decrease the incidence of postoperative pulmonary complications.12 In obese subjects undergoing abdominal surgery, intraoperative RM with high PEEP (≥ 10 cm H2O) failed to decrease the frequency of postoperative pulmonary complications in several RCTs.34-36 A care bundle for obese patients including a RM and titrated PEEP levels intraoperatively and postoperatively might be an option that could be tested.
Adverse effects can occur during RMs,10 but RMs are usually well-tolerated.13,24,27 The RM was prematurely stopped in 10% of subjects as previously reported.23 Blood pressure decreased during the maneuver in our subjects but returned to baseline after the end of the RM, even in the high PEEP group, as previously reported.13,25 A theoretical risk associated with an RM is stretching of a left internal mammary artery graft.25 However, none of our subjects exhibited evidence of cardiac ischemia. Pneumothorax was also uncommon.13,35
The limitations of our study include the failure of randomization to prevent imbalances of some of the variables across the treatment groups. Thus, there was a greater number of mitral surgeries in the 2 RM groups compared to the control group. Mitral surgery carries a higher postoperative complication rate compared to isolated coronary artery bypass graft or aortic valve surgery.37 There was also a higher number of subjects in the 2 RM groups who received blood products. Transfusion of blood products are associated with increased mortality and morbidity.38,39 Therefore, our analysis was adjusted for these imbalances, and RM was still not identified as an independent factor. The lower-than-predicted frequency of postextubation respiratory failure resulted in loss of statistical power and tempered the conclusion. The spontaneous breathing trial with a T-tube might have eliminated any possible treatment effects of PEEP. Our definition of postoperative respiratory failure as the use of any of 3 treatment modalities (ie, invasive mechanical ventilation, BPAP, or HFNC) may be open to criticism. Although predefined criteria for re-intubation were applied, bias cannot be completely ruled out. The intraoperative anesthetic management was not standardized in the protocol. Because the trial was pragmatic, individual PEEP level titration was not performed. A PEEP level of 10 cm H2O, although suggested by some experts, may be insufficient for some obese subjects.13,29,40 Finally, some obese subjects were hypoxemic and likely had significant atelectasis while others did not. Thus, a prophylactic approach and a curative approach coexist in our study, which calls for a cautious interpretation of the results.
Conclusions
The routine use after heart surgery of an RM followed by 5 or 10 cm H2O of PEEP did not decrease the frequency of respiratory failure in obese subjects. A RM followed by 5 cm H2O of PEEP is not appropriate.
Acknowledgments
We thank Mr Stéphane Morisset, biostatistician, for his critical review and supplemental statistical analyses; Dr Aminata Traore for technical assistance; and Dr Antoinette Wolfe for English editing.
Footnotes
- Correspondence: François Stéphan MD, Cardiothoracic ICU, Department of Anesthesiology and ICU, Hôpital Marie Lannelongue, 133 Avenue de la Résistance, 92350 Le Plessis Robinson, France. E-mail: f.stephan{at}ghpsj.fr
Dr Stéphan has disclosed a relationship with Fisher & Paykel Healthcare. The other authors have disclosed no conflicts of interest.
Supplementary material related to this paper is available at http://www.rcjournal.com.
- Copyright © 2021 by Daedalus Enterprises