Abstract
Postoperative pulmonary complications contribute to perioperative morbidity and mortality in addition to being associated with increased health care costs. In this review article, we outline risk factors for the development postoperative pulmonary complications, describe their impact on perioperative outcomes, and focus on the role of intraoperative ventilation strategies in decreasing postoperative pulmonary complications.
Introduction
Ventilator-induced lung injury has been well established in the ICU setting. It has been questioned whether such injury concerns the healthy patient undergoing surgery under general anesthesia exposed to only a few hours of mechanical ventilation. Postoperative pulmonary complications (PPCs) are a major source of perioperative morbidity and mortality1,2 and can significantly increase the cost of hospitalization.3,4
Several studies have addressed the question whether lung-protective ventilator strategies impact the incidence of PPCs. Below we discuss who is at risk for such PPCs and intraoperative protective strategies aimed at reducing perioperative morbidity and mortality associated with these complications.
What Is Lung Injury?
Ventilator-induced lung injury can occur through several mechanisms.5 Traditionally described processes include atelectrauma, volutrauma, barotrauma, and biotrauma, although additional ways may exist. Atelectrauma refers to injury that occurs through cyclic opening and collapse of atelectatic yet recruitable alveoli through the generation of high shear stress at the edge of the collapsed airway and air bolus.5 Volutrauma, or lung injury induced by high tidal volume (VT), occurs through the overdistention of alveoli. Barotrauma, or high-pressure injury, occurs when the lung is exposed to high pressures; of note, airway pressure does not determine the amount of injury, rather it is the transpulmonary pressure (ie, the pressure between the inside and the outside of the lung [pleural space]). Biotrauma refers to lung injury that occurs through inflammatory mediators released in the setting of mechanical injury and can impact lung areas not directly exposed to mechanical injury.
Who Is at High Risk of Lung Injury When Undergoing Surgery?
While scores such as the Lung Injury Prediction Score (LIPS)6 or the Early Acute Lung Injury Score7 aim to identify at-risk patients outside the operating room, preoperative and intraoperative risk factors have been identified for the surgical patient (Table 1).8 Several scores have been developed to predict who is at increased risk. The Assess Respiratory Risk in Surgical Patients in Catalonia (ARISCAT) score9 includes patient risk factors as well as procedural risk factors that have been reported to be sensitive in predicting PPCs (Table 2).10
Factors Associated With Development of Postoperative Pulmonary Complications
Assess Respiratory Risk in Surgical Patients in Catalonia (ARISCAT) Score
What Are PPCs?
In 2015, a European Perioperative Taskforce defined perioperative outcomes including PPCs.11 This composite outcome was defined as one or more of respiratory infection, respiratory failure, pleural effusion, atelectasis, pneumothorax, bronchospasm, aspiration pneumonitis, pneumonia, ARDS, tracheobronchitis, pulmonary edema, exacerbation of preexisting disease, pulmonary embolism, and death. Most authors do not strictly use this definition but only include some of its constituent outcomes, such as respiratory failure and pneumonia.1 Given different methodologies used to define PPCs, it is not surprising that the reported incidence of PPCs varies widely. In a recent randomized controlled trial the incidence reached as high as 39%.12
The morbidity and mortality associated with PPCs is noteworthy. In an observational multicenter study, Fernandez-Bustamante et al13 reported that, in subjects deemed American Society of Anesthesiologists physical status 3, even a single mild PPC, such as the need for nasal cannula, increased length of hospital stay, while more severe PPCs were associated with a significant increase in mortality.13 Khuri et al14 reported that mortality increased by 5- to 10-fold in subjects with PPCs, depending on the type of major noncardiac surgery that was performed. In subjects with PPCs, admission to the ICU increased significantly,13 and length of stay increased by an average of 5 d.15 Not surprisingly, PPCs were associated with a significant increase in costs, likely reflecting the increased length of stay.4 Table 3 summarizes the outcomes of PPCs.
Outcome of Postoperative Pulmonary Complications
What Are the Components of a Lung-Protective Strategy in the Operating Room?
Lung-protective ventilation, consisting of low VT ventilation and adequate PEEP, has been the hallmark of mechanical ventilation in the ICU setting for the last 2 decades. In a large observational study, Ladha et al16 seem to confirm that low VT ventilation in the operating room is protective against the development of PPCs. The authors reported that, of 69,265 enrolled subjects, 34,800 (50.2%) received protective ventilation and 34,465 (49.8%) received nonprotective ventilation intraoperatively. Protective ventilation, defined as PEEP ≥ 5 cm H2O, VT < 10 mL/kg, and a plateau pressure < 30 cm H2O, was associated with a decreased risk of postoperative respiratory complications (adjusted odds ratio 0.90 [95% CI 0.82–0.98], P = .01). A PEEP of 5 cm H2O and median plateau pressures of ≤ 16 cm H2O were associated with the lowest risk of postoperative respiratory failure. A recent meta-analysis of 34 randomized controlled trials including 5,273 subjects concluded that VT < 8 mL/kg and a PEEP of ≥ 5 cm H2O had the lowest risk of developing PPCs.17 However, recent large, randomized controlled trials do not support these findings. Karalapillai et al12 performed a randomized clinical trial in 1,236 subjects undergoing general anesthesia, comparing VT of 6 mL/kg and VT of 10 mL/kg, with PEEP set at 5 cm H2O. The primary end point was development of PPC. The authors defined the composite end point as pneumonia, bronchospasm, atelectasis, pulmonary congestion, respiratory failure, pleural effusion, pneumothorax, or unplanned requirement for postoperative mechanical ventilation, CPAP, or noninvasive or invasive ventilation. The incidence of PPCs did not differ between groups (38% vs 39%).
Like low VT ventilation, high PEEP did not alter the incidence of PPC in a randomized controlled trial conducted in obese subjects.18 The investigators randomized patients with body mass index > 35 kg/m2 to receive PEEP of 5 or 12 cm H2O. Both groups were ventilated with a VT of 7 mL/kg predicted body weight. PPCs occurred in 21.3% of subjects in the high-level PEEP group and in 23.6% of subjects in the low-level PEEP group (95% CI –5.9 to 1.4%, P = .23). Hypoxemia was more common in the low-PEEP group, whereas hypotension and bradycardia were more common in the high-PEEP group. In-hospital mortality was slightly worse in the high-PEEP group but did not reach statistical significance (P = .09).
Individualized Approach to Intraoperative Ventilation
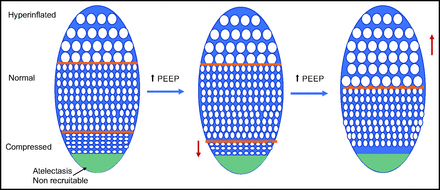
As described above, newer, large, randomized controlled trials of either low VT strategies or high PEEP aiming to reduce PPCs have not reported different outcomes. However, it has been reported that strategies to decrease driving pressure have resulted in reduced incidence of PPCs. In a meta-analysis of 17 trials including 2,250 subjects, Neto et al19 reported that there was a correlation between increased driving pressure and development of PPCs, whereas such a relationship did not exist for PEEP or VT. These findings might not be too surprising given the heterogeneity of ventilation-perfusion mismatch in anesthetized patients.20 Meier et al21 recently discussed that the strategy to optimize driving pressure should depend on the pathophysiology of the lung tissue (example illustrated in Figure 1). A patient-centered individualized approach might therefore offer more success to decrease the incidence of PPCs. Pereira and coworkers22 randomized 40 subjects undergoing abdominal surgery to either receive PEEP of 4 cm H2O or PEEP guided by electrical impedance tomography. These investigators reported significantly lower intraoperative driving pressures, higher intraoperative 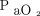 values, as well as reduced postoperative atelectasis in the group receiving electric impendence-guided PEEP. Park et al23 recently reported the results of a randomized controlled trial enrolling 292 subjects undergoing single-lung ventilation for thoracic surgery. The authors used low VT ventilation in both groups and optimized PEEP in the intervention group to achieve the lowest driving pressures. They reported a decrease of severe PPCs (ie, pneumonia, ARDS) from 15% to 6.9% in the intervention group (odds ratio 0.42 [95% CI 0.19–0.92], P = .03). These studies support the hypothesis that a personalized approach to intraoperative ventilation is needed to decrease the incidence of PPC.
values, as well as reduced postoperative atelectasis in the group receiving electric impendence-guided PEEP. Park et al23 recently reported the results of a randomized controlled trial enrolling 292 subjects undergoing single-lung ventilation for thoracic surgery. The authors used low VT ventilation in both groups and optimized PEEP in the intervention group to achieve the lowest driving pressures. They reported a decrease of severe PPCs (ie, pneumonia, ARDS) from 15% to 6.9% in the intervention group (odds ratio 0.42 [95% CI 0.19–0.92], P = .03). These studies support the hypothesis that a personalized approach to intraoperative ventilation is needed to decrease the incidence of PPC.
Individualized PEEP and lung physiology. Increasing PEEP recruits alveoli. If tidal volume remains stable, the driving pressure will fall. Further increase in PEEP causes hyperinflation and increasing driving pressures.
Summary
PPCs are a major contributor to postoperative morbidity and mortality. While retrospective large database studies indicate a benefit of a low VT and moderate to high PEEP strategy, this has not been confirmed in recent randomized controlled trials. An approach focusing on optimizing driving pressures including individualizing PEEP rather than a fixed VT or PEEP strategy are most likely to optimize perioperative pulmonary outcomes.
Footnotes
- Correspondence: Ulrich H Schmidt MD PhD MBA. E-mail: uschmidt{at}health.ucsd.edu
Dr Schmidt presented a version of this paper at the New Horizons Symposium at the AARC Congress 2020 LIVE!, held virtually November 18, 2020.
The authors have disclosed no conflicts of interest.
- Copyright © 2021 by Daedalus Enterprises