Abstract
Background: Ribavirin is an antiviral drug that for many years has been administered to the lungs by aerosolization. Despite advancements in oral delivery routes, there has been a renewed interested in delivering ribavirin via the pulmonary route in select populations. The vibrating mesh micropump (VMM) nebulizer was previously demonstrated to be an effective alternative to the small particle aerosol generator (SPAG) in physicochemical makeup and concentrations of the ribavirin pre and post nebulization. However, the antiviral activity of ribavirin has never been examined. We sought to study ribavirin’s activity pre and post nebulization via VMM.
Methods: We grew and infected human epithelial type 2 cells (HEp2) and primary airway epithelial cells with respiratory syncytial virus (RSV). We then compared the antiviral effects of non-nebulized and aerosolized ribavirin to untreated controls. We used a traditional plaque assay and real-time PCR to determine the quantity of the virus.
Results: Both non-nebulized and nebulized ribavirin reduced the size of RSV plaques compared to untreated controls. Additionally, the non-nebulized and nebulized ribavirin equally inhibited RSV replication. There were no statistically significant differences between non-nebulized and nebulized ribavirin at all time points.
Conclusions: The VMM nebulizer does not affect the antiviral properties of nebulized ribavirin when compared to non-nebulized drug. Our findings add supporting evidence for the use of the VMM nebulizer in the administration of inhaled ribavirin.
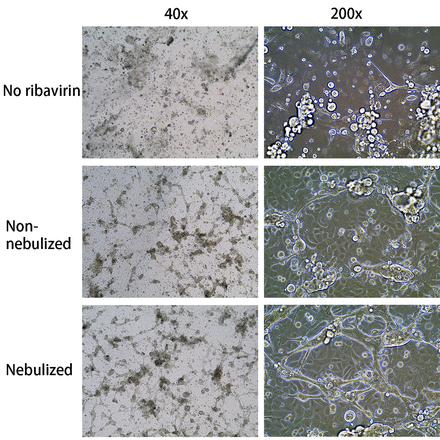
Effect of non-nebulized and nebulized ribavirin in protection against cytopathic effect of RSV on PBECs. A) Cells were infected at MOI of 0.2 for 3 days. Images were taken at 200x. B) Cells were infected at MOI of 0.02 for 5 days. Images were taken at 40x and 200x.
Footnotes
Commercial Relationships: None
Support: This study was funded through an investigator initiated unrestricted research grant provided by Aerogen who is the maker of the Solo nebulizer. To balance the potential conflict of interest we created an agreement that did not restrict the publication of our findings.
- Copyright © 2021 by Daedalus Enterprises