Abstract
BACKGROUND: Whereas high-flow nasal cannula (HFNC) oxygen therapy is increasingly used in patients with exacerbation of COPD, the effectiveness of β2 agonist nebulization through HFNC has been poorly assessed. We hypothesized that salbutamol vibrating-mesh nebulization through HFNC improves pulmonary function tests in subjects with COPD.
METHODS: We conducted a physiological crossover study including subjects admitted to the ICU for severe exacerbation of COPD. After subject improvement allowing a 3-h washout period without bronchodilator, pulmonary function tests were performed while breathing through HFNC alone and after salbutamol vibrating-mesh nebulization through HFNC. The primary end point consisted in the changes in FEV1 before and after salbutamol nebulization. Secondary end points included the changes in FVC, peak expiratory flow (PEF), airway resistance, and clinical parameters.
RESULTS: Among the 15 subjects included, mean (SD) FEV1 significantly increased after salbutamol nebulization from 931 mL (383) to 1,019 (432), mean difference +87 mL (95% CI 30–145) (P = .006). Similarly, FVC and PEF significantly increased, +174 mL (95% CI 66–282) (P = .004) and +0.3 L/min (95% CI 0–0.6) (P = .037), respectively. Airway resistances and breathing frequency did not significantly differ, whereas heart rate significantly increased after nebulization.
CONCLUSIONS: In subjects with severe exacerbation of COPD, salbutamol vibrating-mesh nebulization through HFNC induced a significant bronchodilator effect with volume and flow improvement.
Introduction
COPD is characterized by the occurrence of recurrent acute episodes of exacerbation, with a global burden estimated at about 1.5 million emergency department visits per year in the United States.1 Bronchodilator therapy is the main pharmacologic treatment,2,3 whereas noninvasive ventilation (NIV) is strongly recommended in patients with severe acute hypercapnic respiratory failure as a means of reversing respiratory acidosis and also decreasing work of breathing.4 High-flow nasal cannula (HFNC) therapy has been proposed either as an alternative to NIV in case of poor tolerance5-7 or as an alternative to standard oxygen between NIV sessions.8,9 Indeed, HFNC could be considered as a ventilatory support in patients with COPD, as physiological studies suggest favorable effects on the work of breathing and gas exchange.10-12 HFNC delivers humidified and heated gas through a nasal cannula at high flow that reduces anatomical dead space in the upper airways, clearing exhaled carbon dioxide.13,14 Moreover, the high flow generates a low level of positive pressure in the upper airways, which can provide a slight PEEP effect.15-17 All these physiological effects have been shown to help in decreasing  , breathing frequency, and improving breathing pattern with higher tidal volumes in stable patients with COPD10-12,18,19 as well in unstable patients.17,20,21 A recent noninferiority randomized controlled trial comparing HFNC with NIV reported that HFNC was statistically noninferior to NIV as initial ventilatory support in decreasing
, breathing frequency, and improving breathing pattern with higher tidal volumes in stable patients with COPD10-12,18,19 as well in unstable patients.17,20,21 A recent noninferiority randomized controlled trial comparing HFNC with NIV reported that HFNC was statistically noninferior to NIV as initial ventilatory support in decreasing  after 2 h of treatment in subjects with mild-to-moderate COPD exacerbation.22 Another trial is ongoing and compares the impact of HFNC and standard oxygen (in between 2 NIV sessions) on ventilatory support duration in subjects with COPD exacerbation.8 It appears that HFNC is gradually being used in patients with unstable COPD as an alternative to standard oxygen and even to NIV. Therefore, the delivery of bronchodilator therapy through HFNC may be relevant to management of patients with COPD exacerbation.23,24
after 2 h of treatment in subjects with mild-to-moderate COPD exacerbation.22 Another trial is ongoing and compares the impact of HFNC and standard oxygen (in between 2 NIV sessions) on ventilatory support duration in subjects with COPD exacerbation.8 It appears that HFNC is gradually being used in patients with unstable COPD as an alternative to standard oxygen and even to NIV. Therefore, the delivery of bronchodilator therapy through HFNC may be relevant to management of patients with COPD exacerbation.23,24
An anatomical bench study has previously shown that a vibrating-mesh nebulization of bronchodilator through HFNC was able to deliver relevant masses of aerosol even in cases of high subject inspiratory flow simulation.25 Clinical studies conducted in stable ambulatory subjects with COPD have shown that bronchodilator therapies are effectively delivered within a HFNC circuit and that they provide bronchodilation similar to standard mask jet nebulization.26,27
Accordingly, the objective of this study was to evaluate noninvasively the physiological effects of salbutamol vibrating-mesh nebulization through the HFNC circuit on pulmonary function tests and clinical parameters in subjects admitted in ICU for severe COPD exacerbation.
QUICK LOOK
Current Knowledge
According to its physiological effects, high-flow nasal cannula (HFNC) oxygen therapy is increasingly used in patients with exacerbation of COPD, either as alternative to noninvasive ventilation (NIV) in case of poor tolerance or as an alternative to standard oxygen between NIV sessions. β2 agonist nebulization is the main pharmacologic treatment in patients with severe acute hypercapnic respiratory failure. However, the effectiveness of β2 agonist nebulization through HFNC has been poorly assessed.
What This Paper Contributes to Our Knowledge
This physiological study showed that in subjects with severe exacerbation of COPD salbutamol vibrating-mesh nebulization through HFNC induced a significant bronchodilator effect with volume and flow improvement, suggesting a reduction of dynamic hyperinflation. Therefore, HFNC could be continued without being interrupted during β2 agonist nebulization.
Methods
Study Design and Subjects
This study was a monocenter physiological prospective crossover study, approved by the independent ethics committee of Ile de France (CPP Ile de France XI, 2017–001579-22) and conducted in the ICU of Poitiers University Hospital between January and September 2019. The study was registered on ClinicalTrial.gov, number NCT03449056. Written informed consent was obtained from all subjects before inclusion in the study.
Consecutive adult patients admitted to ICU for COPD exacerbation with respiratory acidosis (arterial pH ≤ 7.35) and requiring NIV4 were screened for eligibility. Underlying COPD could be either documented by spirometry and defined by FEV1/FVC ratio < 0.7028 or highly suspected. Subjects with highly suspected underlying COPD without previous spirometry needed to have a history of smoking and emphysema on chest x-ray or scanner without other reasons for respiratory acidosis. Patients were included after improvement of their respiratory status if they met the following criteria: frequency < 35 breaths/min, Glasgow coma scale score of 15 points, NIV sessions interspaced at least 6 h, and bronchodilator nebulization sessions interspaced at least 3 h. Noninclusion criteria included contraindication to salbutamol and treatment by a β blocker, indication for urgent intubation, hemodynamic or neurologic failure, do-not-intubate order, pregnancy, breastfeeding, no health care insurance, trusteeship, and guardianship. Long-acting bronchodilators were systematically stopped at admission in ICU.
Interventions
After a 3-h washout period without bronchodilator nebulization, subjects received first a 1-h HFNC alone session and then a salbutamol vibrating-mesh nebulization through the HFNC circuit for 30 min.
High-Flow Nasal Cannula Therapy.
HFNC was delivered by a continuous mixture of air and oxygen via binasal prongs, using medium-size cannula, with a gas flow of 30 L/min through a heated humidifier (Fisher & Paykel, Auckland, New Zealand), allowing 100% relative humidity at 37°C and an  to maintain pulse oximetry between 90–92%.
to maintain pulse oximetry between 90–92%.
Nebulization.
Salbutamol was nebulized after reconstitution of 5 mg in 5 mL in a 0.9% isotonic saline solution through a vibrating-mesh nebulizer (Aerogen, Galway, Ireland) placed upstream of the humidification chamber of the HFNC circuit. The session lasted 30 min, and the complete delivery of salbutamol was systematically checked.
Data Collection
Demographic data were collected at inclusion and clinical parameters at baseline at the end of the 1-h HFNC alone session and 40 min after salbutamol vibrating-mesh nebulization through the HFNC circuit. Dyspnea was assessed using a Borg scale ranging from 0–10 points, a higher score indicating maximal dyspnea; and subject comfort was recorded using a visual numeric scale ranging from 1–5 points, ie, very uncomfortable to very comfortable.
All pulmonary function tests were performed using a spirometer (Vyaire Medical, Chicago, Illinois) with dedicated software (The Surgical Company France, Flaxlanden, France) at baseline, at the end of the 1-h HFNC alone session, and 40 min after salbutamol vibrating-mesh nebulization through the HFNC circuit. HFNC was removed from the subject during the pulmonary function test.
At each time, 2 flow-volume loops and a minimum of one slow spirometry were recorded. The flow-volume loop with the best value of peak expiratory flow (PEF) was selected for analysis. Mean airway resistance values were computed after 10 values recorded by an automatic occlusion procedure. The spirometry procedure was performed following the American Thoracic Society/European Respiratory Society guidelines for the standardization of lung function testing.29
Outcomes
The primary outcome consisted in changes in FEV1 after salbutamol vibrating-mesh nebulization through the HFNC circuit. Secondary outcomes included changes in other spirometry parameters, FVC, PEF, slow vital capacity (slow VC), mean airway resistance; and clinical parameters, breathing frequency, heart rate, and dyspnea level.
Statistical Analysis
On the basis of a mean difference in FEV1 of 200 mL before and after salbutamol nebulization with SD of 200 mL and according to the crossover design, we calculated that an enrollment of 16 subjects was required to provide the study a power of 0.8 at a 2-sided alpha level of 0.05. Quantitative variables were expressed as median and interquartile range (IQR) or mean and SD when normally distributed. Mean differences were compared before and after nebulization using a t test. A P value < .05 was considered as significant. All analyses were performed using Prism software (version 7.1) (GraphPad Software, San Diego, California).
Results
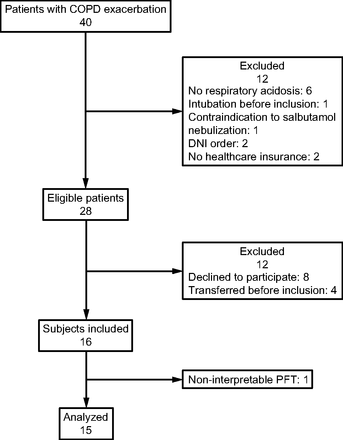
Among the 40 patients with COPD exacerbation screened for eligibility, 28 were eligible, 16 were included from January 2019 to June 2021, but one subject was secondarily excluded for noninterpretable pulmonary function tests (Fig. 1). Among the 15 subjects studied, 13 (87%) had confirmed COPD by previous respiratory function tests including 4 subjects with severe COPD according to the GOLD classification (Table 1). Median FEV1 was 58% (IQR 41–73%) expressed in percentage of predicted value according to sex and age. The interval from ICU admission to inclusion was 5 d in median (IQR 3–8), and 14 out of 15 subjects were treated with NIV.
Flow chart. DNI = do not intubate. PFT = pulmonary function test.
Population Characteristics at Baseline
Outcome
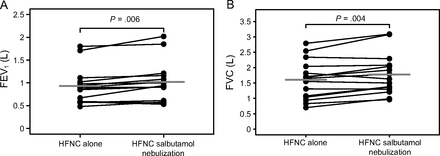
Mean (SD) FEV1 increased from 931 mL (SD 383) after the 1-h HFNC alone session to 1,019 mL (SD 432) after salbutamol vibrating-mesh nebulization through HFNC circuit, mean difference 87 mL (95% CI 30–145) (P = .006) (Table 2). FEV1 increased in 80% of subjects (12 out of the 15 subjects) (Fig. 2). Similarly, mean FVC increased from 1,601 mL (SD 633) to 1,775 mL (SD 661), mean difference 174 mL (95% CI 66–282) (P = .004), and PEF from 2.9 L/min (SD 1.4) to 3.2 L/min (SD 1.2), mean difference 0.3 L/min (95% CI 0–0.6) (P = .037), after salbutamol vibrating-mesh nebulization. FVC increased in 11 subjects (73%) and PEF in 10 subjects (67%) (Table 2 and Fig. 2). Mean airways resistance did not reduce significantly after nebulization, mean difference −0.6 (95% CI −1.5–0.3) (P = .19).
Spirometry Parameters Before and After Salbutamol Nebulization Through HFNC Circuit
A: Absolute individual changes in FEV1 after high-flow nasal cannula (HFNC) therapy alone and after salbutamol vibrating-mesh nebulization through HFNC circuit; gray lines indicate mean values. B: Absolute individual changes in FVC after HFNC therapy alone and after salbutamol vibrating-mesh nebulization through HFNC circuit; gray lines indicate mean values.
No difference was observed in breathing frequency, pulse oximetry, or blood pressure, whereas heart rate increased significantly after salbutamol nebulization (P < .01) (Table 3). The median score on the Borg scale after nebulization did not change significantly, 1.0 (IQR 0–3) to 0.5 (IQR 0–2) (P = .063).
Changes in Clinical Parameters Before and After Salbutamol Nebulization Through HFNC Circuit
Tolerance
Comfort evaluated by visual numeric scale was similar during HFNC session and during nebulization, 5.0 (IQR 4–5) and 5.0 (IQR 4–5) (P > .99), respectively. No serious adverse event related to HFNC or salbutamol vibrating-mesh nebulization was observed during procedures. Tremors related to salbutamol administration occurred in 2 subjects, with rapid spontaneous resolution.
Discussion
In this physiological crossover study, subjects with COPD exacerbation significantly improved their FEV1, FVC, and PEF after salbutamol vibrating-mesh nebulization through HFNC as compared to HFNC alone, suggesting an effective bronchodilator effect. The decreased resistance of airways did not reach significance but was in line with the bronchodilator effect. Lastly, the significantly increased heart rate also suggested a systemic passage of β2 agonist after nebulization.
The physiological effects of HFNC10-1,2,17-21 and benefits reported in critically ill patients30,31 have favored its use in patients with hypercapnic respiratory failure or COPD exacerbation.6,7,32 That is one reason why physicians may be confronted increasingly with patients having HFNC in place of standard oxygen6,7 or during breaks of NIV9 and requiring inhaled bronchodilator therapy. The application of HFNC may facilitate clearance of carbon dioxide through a high flow of gas that promotes washout and ventilation of the anatomical dead space of the upper airways.13,14 The PEEP generated by the system may facilitate the decrease of work of breathing in patients with air flow obstruction by counterbalancing flow-limited intrinsic PEEP.15,16 These physiological effects help to reduce inspiratory effort and neuroventilatory drive in stable11,33 or unstable patients with COPD.10 Accordingly, the application of bronchodilator therapy through the HFNC system could be an option to avoid interruption of HFNC during management of patients with COPD exacerbation.
The optimal configuration for nebulization through the HFNC system has been shown to be placement immediately upstream before the humidification chamber with a gas flow not exceeding 30 L/min.25,26 Indeed, the mechanisms and properties of the HFNC system may interfere with nebulized drug delivery. First, the high gas flow and subsequent turbulent flow and the shape angulation of the nasal cannula may favor impaction of drug particles in the circuit. The high gas humidity may lead to increased particle sizes and reduce the fraction of aerosol made of particles with the optimal size (0.5–5.0 µm).34 Last, the nose anatomy physiologically retaining inhaled particles is a barrier to efficient drug delivery after nebulization. However, a recent physiological study has shown that albuterol delivered by vibrating-mesh nebulization through an HFNC circuit appeared noninferior to standard face mask jet nebulization on pulmonary function tests.26 Moreover, the difference in inhalable mass at the cannula outlet did not seem to depend on the choice of the nebulizer (ie, jet nebulizer connected to a bucco-nasal oronasal mask or vibrating-mesh nebulizer connected to humidification chamber of the HFNC system).26 Accordingly, we chose to perform nebulization through the HFNC system with a vibrating-mesh nebulizer positioned upstream of the humidification chamber and at a gas flow of 30 L/min in order to benefit from the physiological effects of HFNC and the optimal nebulization conditions.
FEV1 and FVC are reliable parameters to describe change in air flow limitation or volume retention, as they have been shown to be highly reproducible in a large proportion of patients, provided that they are obtained by well-trained technicians.35 In our study, salbutamol nebulization through the HFNC circuit increased FEV1 (primary outcome), but this did not reach the usual criteria of reversibility (ie, a 12% and/or 200 mL increase).36 A recent study showed that the prevalence of bronchodilator reversibility in subjects with COPD was only 17% when these usual criteria were met.37 However, a change of 5–10% of FEV1 from baseline values is considered as clinically relevant, whereas a change below 3% has been deemed not to be.35 Therefore, a slight increase in FEV1 can result in a reduction in residual volume and delay the onset of dynamic hyperinflation during exercise and tachypnea.2,38,39 Similarly to our study, Braunlich and Wirtz reported27 in 26 nonselected stable subjects with COPD a 9.4% increase in FEV1 30 min after bronchodilator (salbutamol and ipratropium) nebulization using a jet nebulizer adapted on a HFNC system. In ambulatory subjects with a known reversible obstructive pulmonary disease, Reminiac et al26 highlighted a greater increase of 16% of FEV1 using a vibrating-mesh nebulizer through HFNC system.
We reported increased FVC after salbutamol nebulization, which could be considered as the consequence of a reduction in lung hyperinflation.40,41 It has been shown in cohort studies including subjects with COPD that a response to bronchodilator therapy could be better detected by performing FVC rather than FEV1.40,41 Indeed, improvement of FVC after bronchodilator administration is related to reduction in residual volume. It results in an increased inspiratory capacity, which better reflects reduction in lung hyperinflation during COPD exacerbation.40,41 In our study, improvement in FVC may, therefore, reflect a volume response to bronchodilator therapy, suggesting a reduction of dynamic hyperinflation in our population. However, inspiratory capacity was not evaluated to confirm this hypothesis. Moreover, we found no change in slow VC, despite increased FEV1 and FVC after salbutamol nebulization. This could be explained by the fact that the expiratory time required to perform a slow VC is longer than that required to perform an FVC. Therefore, it favors the complete emptying of lungs regardless of the period before or after bronchodilator treatment.
One limitation is learning effect due to repeated spirometry procedures that was not controlled by randomized record order. However, most subjects had previously performed pulmonary function tests, suggesting a small impact of this effect on measurements. Second, 2 flow-volume loops were performed rather than 3 (as recommended in stable patients), and only performed under HFNC and after nebulization through HFNC system, in order to avoid exhaustion of subjects who were still recovering from an episode of COPD exacerbation. Lastly, the extrapolation of effective nebulization through HFNC circuit to other drugs cannot be established, as it has been reported that lung deposition using this nebulization route was below 1%, suggesting an observed effect also due to the high therapeutic index of salbutamol.25
Conclusions
In subjects with severe COPD exacerbation, salbutamol nebulization using vibrating-mesh nebulizer through HFNC circuit induced significant but moderate bronchodilation with decreased FEV1 and PEF. Moreover, improvement of FVC suggests a reduction of dynamic hyperinflation.
Acknowledgments
The authors wish to thank Jeffrey Arsham for reviewing and editing the original English language manuscript.
Footnotes
- Correspondence: Jean-Pierre Frat MD PhD, Médecine Intensive Réanimation, Centre hospitalier universitaire de Poitiers, 2 rue de la Milétrie, CS 90577, 86021 Poitiers Cedex. E-mail: jean-pierre.frat{at}chu-poitiers.fr
See the Related Editorial on Page 149
The study was performed at intensive care of Poitiers University Hospital: CHU de Poitiers, Médecine Intensive Réanimation, Poitiers, France.
The study received a grant from Le nouveau souffle.
The study was registered on ClinicalTrial.gov: NCT03449056.
Dr Frat discloses relationships with the French Ministry of Health, Fisher & Paykel Healthcare, and SOS Oxygène. Dr Thille discloses relationships with the French Ministry of Health, Fisher & Paykel Healthcare, Maquet Getinge, GE Healthcare, and Covidien. Dr Marjanovic discloses a relationship with Fisher & Paykel. The remaining authors have no conflicts to disclose.
- Copyright © 2022 by Daedalus Enterprises