Abstract
BACKGROUND: Asthma is a heterogeneous disease, usually characterized by chronic airway inflammation. Although inspiratory muscle training (IMT) and high-intensity interval training (HIIT) are beneficial for patients with asthma, controversies persist. Therefore, we aimed to investigate the effects of IMT and HIIT on lung function and respiratory muscle function of subjects with asthma.
METHODS: We searched PubMed, Embase, Web of Science, and the Cochrane Library databases up to May 2021. Inclusion criteria were randomized controlled trials (RCTs) of subjects with asthma who received either IMT or HIIT. The outcome measures were changes in lung function and respiratory muscle function.
RESULTS: A total of 13 RCTs (10 in IMT and 3 in HIIT) were included, with a total of 598 subjects. The meta-analysis showed a significantly improved FEV1 of the expected value (FEV1%pred) (mean difference [MD] 4.49% [95% CI 2.31–6.67], P < .001; I2 = 13%), FVC of the expected value (FVC % pred) (MD 5.72% [95% CI 3.56–7.88], P < .001; I2 = 0%), FEV1/FVC % (MD 5.01% [95% CI 2.45–7.58], P < .001; I2 = 25%), FVC (L) (MD 0.21 L [95% CI 0.03–0.40], P = .02; I2 = 0%), maximum inspiratory pressure (PImax) (MD 27.62 cm H2O [95% CI 6.50–48.74], P = .01; I2 = 96%), and PImax (%pred) (MD 27.35% [95% CI 6.94–47.76], P = .009; I2 = 83.5%) in the IMT group. There was no statistical significance in maximum expiratory pressure.
CONCLUSIONS: IMT improved pulmonary function (FEV1%pred, FVC) and inspiratory muscle strength in subjects with stable asthma. Due to the small number of RCT studies included and the limited outcome measures involving HIIT, we were unable to draw conclusions about whether HIIT was beneficial in this meta-analysis. Moreover, clinical heterogeneity exists in different areas such as population and training programs; the above conclusions still need to be confirmed in future studies.
Introduction
Asthma is a disease characterized by chronic airway inflammation and airway hyper-reactivity leading to cough, wheeze, shortness of breath, chest tightness, together with variable expiratory air flow limitation.1 Asthma affects approximately 300 million people worldwide, with an additional 100 million likely to be affected by 2025.1,2 The global prevalence rates of self-reported, doctor-diagnosed asthma range from 1–18% in different countries.1,3,4 Asthma causes substantial disability, impaired quality of life, and avoidable deaths in children and young adults and imposes great burden not only on the individual and their family but also leading to a huge economic loss.3
Most patients may acquire clinical control of their asthma with adequate pharmacotherapy, typically by inhaled medication. However, because of the chronic nature of the disease and the requirement of prolonged medication, non–pharmacologic treatment modalities have also been tried in adults and children.5-7 To date, several studies have shown that inspiratory muscle training (IMT) and high-intensity interval training (HIIT) method improve cardiopulmonary fitness, asthma symptoms, and quality of life in subjects with asthma.8-12
IMT and HIIT are promising asthma treatments that are used by a wide range of patients. IMT is an intervention that has been found to enhance the strength and endurance of inspiratory muscles in various populations by using devices or equipment to apply resistance to inspiration.13-18 Patients with asthma can have reduced respiratory muscle performance due to increased airway resistance. The reason for adopting IMT in patients with asthma is to increase inspiratory muscle function to balance the ventilatory demand and ventilatory capacity connection.10,16 Several emerging studies show that strengthening the inspiratory muscles in people with asthma reduces the severity of their dyspnea and improves their exercise capacity.19-21 A published systematic review showed that IMT can significantly increase maximum inspiratory pressure (PImax).9 HIIT is widely used to improve athletic ability due to its excellent training effect. HIIT involves repeated bouts of relatively higher-intensity exercise interspersed with periods of lower-intensity recovery.22 HIIT has been shown in studies23,24 to enhance asthma control, and people with asthma found it to be tolerable25 and less likely to cause bronchoconstriction.26 Although there have been a number of published studies evaluating the effectiveness of IMT and HIIT methods in patients with asthma, there is still controversy and no consensus on the efficacy of HIIT and IMT in subjects with asthma. Therefore, we performed a meta-analysis of existing evidence to verify the effects of IMT and IMT or HIIT on lung function and respiratory muscle function in patients with asthma.
Methods
We present the following article in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses reporting checklist.
Search Strategy
PubMed, Embase, Web of Science, and the Cochrane Library were all searched from their inception until May 14, 2021. The search strategy in PubMed is described in Table S1 (see related supplementary materials at http://www.rcjournal.com). The same search strategy was used in Embase, Web of Science, and the Cochrane Library based on different specific requirements. There were no restrictions on age or publication date. We searched the following key words: “asthma,” “bronchial asthma,” “inspiratory muscle training,” “high-intensity interval training,” “sprint interval training,” and “exercise, high-intensity intermittent.”
Selection Criteria
We regarded studies to be eligible for inclusion if they met the following criteria: the clinical diagnosis of asthma according to the Global Initiative for Asthma (GINA) recommendations or made by a physician and/or using objective criteria such as bronchodilator reversibility or both; IMT or HIIT should be applied to the experimental group; and randomized controlled trials (RCTs) published in English. And at the end of the intervention period, studies had to include one of the following outcomes: lung function (FEV1% pred, FVC, FVC % pred, FEV1/FVC), PImax, and maximum expiratory pressure (PEmax).
The following studies were excluded: animal studies, studies without subjects with asthma, reviews, guidelines, letters, commentaries, non-RCTs, and duplications. Studies from which data could not be obtained from the original manuscript or an e-mail to the authors were excluded.
Two authors independently assessed the rationality of the included articles. We initially assessed the titles and abstracts of the studies before retrieving the studies that were deemed eligible for full-text review. Any disagreements between the 2 authors were resolved by consulting with a third author.
Data Extraction
Data were extracted from each selected study by 2 authors independently, including general information of the study (author, year, country), population information (number completed, age range [mean]), intervention, and outcomes reported. When the extraction was completed, the 2 authors cross-checked their data. When there was inconsistency in the results, they were discussed with the third author.
Risk of Bias Assessment
Two authors (Wang Q, Yang F) independently evaluated the quality of each selected study using the Cochrane collaboration tools in following 7 aspects:27 random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases.27 Each domain was assigned a high, low, or unclear level of evaluation.27 When there was a disagreement, it was resolved by consultation or with the assistance of a third author.
Statistical Analysis
The statistical analysis was performed using the RevMan software (ReviewManager Version 5.3, Cochrane, London, England, United Kingdom) and the Stata 15.0 software (StataCorp, College Station, Texas). When data from 2 or more studies were available, outcomes were pooled using mean differences (MDs) for continuous variables. Forest plots were used to characterize the pooled effect size, and a P value < .05 was considered statistically significant. The heterogeneity of the studies was assessed using I2 and chi-square statistics, and a fixed-effects model was used to combine the effect size when I2 ≤ 50% or P > .1 in the chi-square statistics. We also attempted to find potential sources of heterogeneity through subgroup analysis or sensitivity analysis if significant heterogeneity (I2 > 50%) emerged using a random-effects model. Funnel plots were quantified to detect any publication bias (P value < .05) if there were sufficient articles.
Results
Study Selection
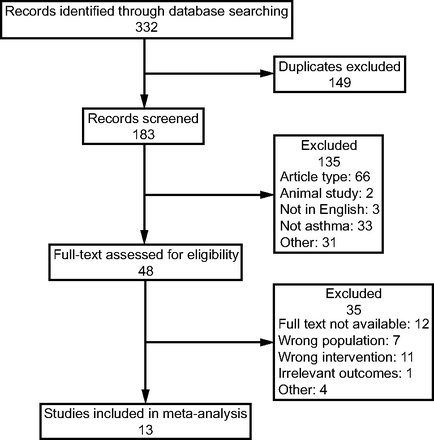
We identified 332 studies using our search strategy. A total of 149 duplicate studies were removed. After evaluating the titles and abstracts, 48 potentially relevant studies were identified. Finally, 13 studies matched our inclusion criteria after reviewing the full text.19-21,23-25,28-34 Figure 1 depicts a flow chart of the study selection process.
Flow chart.
Table 1 illustrates the characteristics of the included studies and participants. There were 13 studies with a total of 598 participants that were published between 1992–2021, with 10 studies involving 356 participants performing IMT19-21,28-34 and 3 studies involving 242 participants performing HIIT.23-25 Of all the papers, 236 patients in 3 articles25,28,31 were children.
Basic Characteristics of the Included Studies
IMT was performed 5 d per week in 3 studies, twice daily in 2 studies, 6 sessions (30 min) per week in 2 studies, and 3 times per week in 2 studies. The training ranged from 6 weeks–6 months, and intensity of IMT ranged from 40–80% of PImax.
IMT was used alone in 8 of 10 studies.19-21,29-31,33,34 When IMT was used alone, it was compared to sham IMT or education. In one study,32 IMT was compared to breathing exercises (diaphragmatic breathing and pursed-lip breathing). In another study,28 both the IMT and placebo groups received the conventional respiratory rehabilitation program, which included abdominal breathing, lip-contraction breathing training, resistance breathing training, breathing training at the end of inhalation pause, systemic breathing exercises, and unconventional breathing training such as relaxation training and multiple breathing training.
The HIIT training mode in one study was spinning cycles, but the other 2 trials did not identify a type of exercise. Furthermore, the frequency of IMT was 3 times per week; the duration of the training ranged from 8 weeks–6 months, and the exercise training sessions ranged from 30–60 min. The exercise intensity was conducted at an intensity equivalent to ≥ 90% of the maximum heart rate or maximum oxygen uptake in the 3 trials.
Risk of Bias in the Included Studies
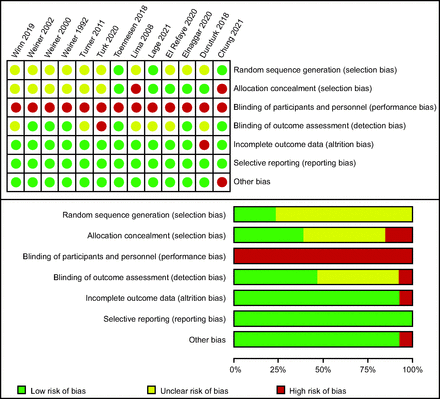
Figure 2 depicts the risk of bias in the included studies. Three studies23,32,34 reported random sequence generation, 5 studies23,28,29,33,34 stated the allocation concealment process, and all of the studies were estimated to have a low risk of reporting bias. However, for blinding of participants and personnel, the studies were all judged as high risk because the design of the study itself. Six studies20,21,23,28,30,32 specified blinding of outcome assessment. One study with a high incidence of withdrawal was considered high risk in the domain of incomplete outcome data.33 Meanwhile, in the domain of other biases, all the studies were deemed to have a low risk except for one study(lack of a no-treatment control group).32
Judgments about each risk of bias item.
Results of the Meta-Analysis
Across the 13 studies included in the qualitative synthesis, 4 outcomes related to lung functions were assessed in percent of predicted values (%pred) or absolute values, including FEV1%pred,19,23-25,28,30,32-34 FVC,19,29,33 FVC %pred,19,23,25,28,30,32-34 and FEV1/FVC.28,29,33
FEV1%pred.
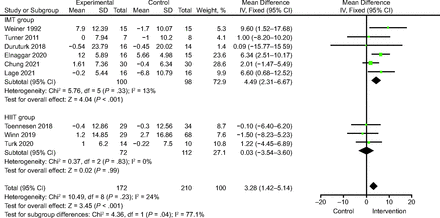
Nine studies19,23-25,28,30,32-34 involving 382 patients reported FEV1% pred. The analysis showed a significant increase in FEV1%pred in the IMT group compared with the control group (MD 4.49% [95% CI 2.31–6.67], P < .001) (Fig. 3). There was no significant heterogeneity (I2 = 13%, P = .33). There was no significant difference between the HIIT and control groups (MD 0.03% [95% CI −3.54 to 3.60], P = .99; I2 = 24%) (Fig. 3).
Forest plots of FEV1%pred: inspiratory muscle training (IMT) versus control and high-intensity interval training (HIIT) versus control.
FVC % pred.
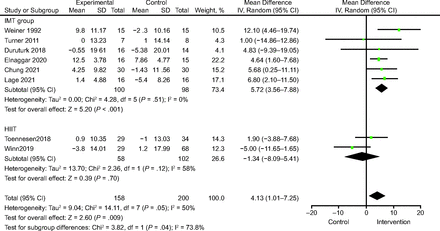
Eight studies19,23,25,28,30,32-34 with 358 participants reported FVC % pred. The analysis indicated a significant improvement in FVC % pred in the IMT group compared to control (MD 5.72% [95% CI 3.56–7.88], P < .001) (Fig. 4) and no significant heterogeneity between studies (I2 = 0%, P = .51). There was no significant difference between the HIIT and control groups (MD −1.34% [95% CI −8.09 to −5.41], P = .70; I2 = 58%) (Fig. 4).
Forest plots of FVC % pred.
FVC.
In 3 trials including 85 participants who received IMT, we utilized a fixed-effects model to assess the pooled impact of FVC.19,29,33 The meta-analysis demonstrated a significant difference in favor of IMT when compared to control groups (MD 0.21 L [95% CI 0.03–0.40], P = .02) (Figure S1, see related supplementary materials at http://www.rcjournal.com). There was no indication of heterogeneity (I2 = 0%).
FEV1/FVC%.
FEV1/FVC% was reported in 3 articles,28,29,33 and a fixed-effects model (I2 = 25%, P = .27) revealed a significant difference in favor of IMT when compared to the controls (MD 5.01% [95% CI 2.45–7.58], P < .001) (Figure S2, see related supplementary materials at http://www.rcjournal.com).
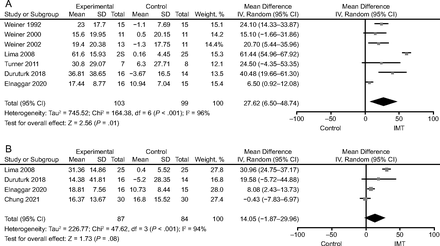
PImax.
Seven studies19-21,28,30,31,33 used PImax reporting in absolute values as the outcome. A random-effect model (I2 = 96%, P < .001) of 7 studies including 202 participators indicated a significant increase of PImax after IMT compared with control groups (MD 27.62 cm H2O [95% CI 6.50–48.74], P = .01) (Fig. 5A).
Forest plots comparing inspiratory muscle training (IMT) and control regarding: A: maximum inspiratory pressure (PImax), B: maximum expiratory pressure (PEmax).
PImax (%pred).
Three studies32-34 used PImax assessed in %pred as the outcome. A pooled random-effects model demonstrated a significant improvement in IMT groups (MD 27.35% [95% CI 6.94–47.76], P = .009; I2 = 84%) (Figure S3, see related supplementary materials at http://www.rcjournal.com).
Sensitivity Analysis
Sensitivity analysis of primary outcomes was conducted by Stata (version 15.0) software. After removing each study, the data revealed that our findings were consistent with the full analysis for all end points.
Discussion
The main purpose of this study was to evaluate the effects of IMT and HIIT on lung function and inspiratory muscle strength in patients with asthma. This meta-analysis confirmed the effectiveness of IMT in improving partial spirometry parameters (FEV1%pred, FVC, FEV1/FVC) and increasing inspiratory muscle strength.
Pulmonary rehabilitation in patients with asthma and COPD overlap is considered an important component in addition to medication and is recommended in the GINA guidelines.1 According to the guideline, physical activity is encouraged for its general health benefits, and breathing exercise and IMT may be useful for asthma.1 Based on empirical evidence, people with asthma are less interested in physical activity and are more likely to participate in lower-intensity aerobic exercises when they do exercise.35 On the other hand, physical inactivity has been associated with negative health consequences and an increase in asthma-related35,36 complications. One previous systematic review and meta-analysis indicate that high physical activity levels are a possible protective factor against asthma development.12
There is evidence that IMT could improve lung function, respiratory muscle strength and endurance, ability levels, and physical activity, as well as reduce emotional disorders, and the use of medical services.37,38 Studies39-46 have shown that inspiratory muscle fatigue causes the so-called metabolic reflex or metaboreflex, resulting in vasoconstriction of the blood vessels in the peripheral muscles, which leads to a decrease in respiratory performance. IMT might minimize the effects of the activation of the inspiratory muscle metaboreflex.47 Furthermore, Lima et al31 found improvement in respiratory symptoms, activities of daily living, and a decrease in the frequency of asthma attacks and medication usage in the IMT group. In contrast, David et al48 found no clinical changes in the group that received IMT.
HIIT has received considerable attention in recent years because it has been identified as a time-efficient method of exercise that can elicit significant improvements in both cardiorespiratory fitness and body composition in youth,49-51 irrespective of whether participants had asthma.25 However, a study discovered that HIIT did not result in significant improvements in lung function, asthma control, or quality of life.25 There was no agreement among the researchers on whether and how these physical therapies benefit patients with asthma.
Our study included 2 interventions (IMT and HIIT) and a meta-analysis. In terms of lung function, our meta-analysis found that IMT significantly increased FEV1% pred, FVC % pred, and FEV1/FVC in subjects with asthma. FEV1 is commonly used to investigate variable expiratory air flow limitation in asthma.3 It represents airway patency and is used to determine airway function and respiratory muscle strength to indicate the degrees of airway obstructions.52,53 The FEV1/FVC is also a useful index for diagnosing airway obstruction in adults.54 Both FVC and FVC % pred are force-dependent parameters reflecting pulmonary capacity and are mainly affected by respiratory muscle strength, lung compliance, and airway resistance. It is possible that, as a result of IMT, the diaphragm and respiratory muscles were able to perform more work, which resulted in an improvement in respiratory capacity and an increase in thoracic expansion and, therefore, had a role in increasing lung volumes.15,16
Ten trials performing IMT were included in the meta-analysis. The trials incorporated a similar intervention protocol, with participants using the pressure-threshold loading device at loads of at least 40% of their PImax. The impact of IMT on asthma was consistent throughout the included studies in the meta-analysis, with all demonstrating an improvement in inspiratory muscle strength (PImax). These improvements were observed across a wide spectrum of populations with various degrees of asthma severity. The pooled results revealed credible improvement in PImax after IMT in subjects with asthma, whereas no statistically significant difference was detected on PEmax, which is consistent with the results from a 2013 Cochrane review by Silva et al.38 In the pediatric population, a meta-analysis was conducted in support of increasing PImax during IMT, although the PEmax findings were controversial.37 The possible explanations are described below. IMT may elicit subtle improvements in airway diameter and air flow limitation during exercise, thereby reducing dynamic hyperinflation and increasing exercise capacity.33 Pulmonary hyperinflation is a pathophysiological manifestation in asthma leading to airway obstruction. It occurs when small-caliber airways close prematurely, increasing the effort of the respiratory muscles at the end of exhalation. It causes the lowering of the diaphragmatic dome, creating a mechanical disadvantage and leading to respiratory muscle weakening.9,55 Training the respiratory muscles and increasing the pressures in the airways may result in less lung hyperinflation, offsetting the functional weakening of the inspiratory muscles, which can alleviate some of the negative effects of dynamic hyperinflation.32 Moreover, during IMT, inspiration and expiration are active throughout the respiratory cycle, promoting muscle function optimization, as evidenced by increased muscle strength.56 Specifically, according to mechanism studies using both human and animal models, IMT has been shown to increase diaphragm thickness56-59 and the proportion of type II muscle fibers in the external intercostal muscles,60 which are accessory muscles of inspiration. Increases in muscle fiber cross-sectional area are generally associated with increased muscle strength.61,62 Our findings support the efficacy of IMT as a management strategy for asthma that could be a complementary strategy to traditional treatments.
This study has some limitations that should be noted. First, clinical characteristics of subjects with asthma were varied, such as age, sex, body mass index, and severity of asthma. Moreover, clinical heterogeneity exists due to the diversity of the intervention designs. More specifically, the training duration ranged from 6 weeks–6 months; the length of each session ranged from 20–60 min, and the frequency ranged from 2–6 sessions per week, all of which might contribute to potential confounders for accurate inclusions. Second, our meta-analysis included data from a small number of trials that included subjects with clinically stable asthma. Therefore, it should not be generalized to patients with acute asthma.
Conclusions
Overall, the analysis demonstrated that IMT improved pulmonary function (FEV1%pred, FVC) and inspiratory muscle strength in stable subjects with asthma. Due to the small number of RCT studies included and the limited outcome measures involving HIIT, we were unable to draw conclusions about whether HIIT was beneficial in this meta-analysis. Moreover, clinical heterogeneity exists in different areas such as population and training programs; the above conclusions still need to be confirmed in future studies. And future research should strive to create a standardized training program based on comparisons of various IMT and HIIT protocols.
Acknowledgments
We would like to thank the researchers and study participants for their contributions.
Footnotes
- Correspondence: Wei Gao MD, Department of Respiratory and Critical Care Medicine, China Rehabilitation Research Center, Rehabilitation School of Capital Medical University, No.10 Jiaomen North Road, Fengtai District, Beijing 100068, China. E-mail: rhhuxi{at}163.com
The authors have disclosed no conflicts of interest.
Supplementary material related to this paper is available at http://www.rcjournal.com.
- Copyright © 2022 by Daedalus Enterprises