Abstract
BACKGROUND: Neurally-adjusted ventilatory assist (NAVA) improves patient-ventilator synchrony and reduces the risk of respiratory over-assistance. Variable pressure support ventilation (PSV) is a recently introduced mode of assisted ventilation that has also shown reduction in patient-ventilator asynchronies. We hypothesized that NAVA would reduce patient-ventilator asynchronies and inspiratory effort compared to variable PSV because breathing variability was intrinsically determined by the patient and not by the ventilator. This study aimed to evaluate patient-ventilator asynchronies and inspiratory effort pressure-time product (PTP) between NAVA and variable PSV in subjects with mild ARDS.
METHODS: After 24 h of controlled mechanical ventilation, subjects (PaO2/FIO2 200–300 and PEEP level < 10 cm H2O) were randomized in sequence 1:1 by using a web-based encrypted platform and assigned to NAVA or variable PSV groups. Both modes of ventilation were consecutively kept for 24 h unless there were clinical changes. The primary aim of this study was to evaluate differences in asynchrony index (AI) between variable PSV and NAVA. Our secondary aims were to evaluate the coefficient of variation (CV) of breathing patterns and inspiratory effort between the groups.
RESULTS: Thirteen subjects were randomized in the NAVA group and 13 subjects in the variable PSV group. AI over time and minute PTP (PTPmin) were not different between NAVA and variable PSV groups (AI t0 P = .52, AI t12 P = .27, AI t24 P = .12; and PTPmin-t0 P = .60, PTPmin-t12 P = .57, PTPmin-t24 P = .85, respectively). CV for tidal volume (VT) and pressure support (PS) was lower in variable PSV group over time compared with NAVA group (P < .05).
CONCLUSIONS: In this randomized controlled trial including subjects with mild ARDS, NAVA and variable PSV had comparable effects on patient-ventilator synchronies and PTP. However, variable PSV reduced the variability of VT and PS when compared with NAVA.
- mechanical ventilation
- ARDS
- pressure support ventilation
- neurally adjusted ventilatory assist ventilation
Introduction
The efficacy and safety of mechanical ventilation are a cornerstone of managing ARDS, and ventilator settings depend on the severity of ARDS.1 In critically ill patients with ARDS, volume-controlled continuous mandatory ventilation (VC-CMV) improves gas exchange and reduces respiratory fatigue.2 However, VC-CMV is often associated with increased use of sedation and neuromuscular blocking agents, which induces diaphragm atrophy by decreasing diaphragmatic efficiency even after 12–24 h.3-7 Spontaneous assisted modes of mechanical ventilation are well tolerated and reduce both adverse effects of prolonged sedation and ventilator-associated diaphragmatic dysfunction.8,9 Neurally-adjusted ventilatory assist (NAVA) is a continuous spontaneous mode of ventilation with servo targeting that uses the electrical activity of the diaphragm (EAdi) to trigger and cycle the inspiratory assistance, providing it in proportion to the patient’s effort.10 The flexibility of NAVA and the link that it establishes between neural breathing control and ventilatory assistance may improve the patient’s ability to tolerate mechanical ventilation during the early phase of partial ventilator support. Different studies showed that NAVA improves patient-ventilator synchrony and reduces the risk of over-assistance, making it an attractive alternative for patients experiencing clinically important asynchrony on pressure support ventilation (PSV).11 PSV is a pressure-controlled mode of spontaneous breathing in which each breath is initiated by the patient (flow or pressure triggered) but supported by constant pressure inflation set by the operator.12 To our knowledge, there are not previous studies that have compared NAVA with variable PSV. Variable PSV is a recently introduced mode of assisted ventilation that increases the variability of tidal volume (VT) and breathing frequency by an extrinsic variation of pressure support (PS), mainly determined by the ventilator13 and has been shown to reduce patient ventilator asynchronies.14 We hypothesized that NAVA was better associated with reduced patient-ventilator asynchronies and inspiratory effort than variable PSV since breathing variability was intrinsically determined by the patient and not by the ventilator. This study aimed to evaluate patient-ventilator asynchronies, variability of breathing patterns, and inspiratory effort (pressure-time product [PTP]) between variable PSV and NAVA in subjects with mild ARDS.
QUICK LOOK
Current Knowledge
In critically ill patients, asynchronies may be associated with negative outcomes. Asynchrony index greater than 10% is associated with increased duration of mechanical ventilation and the use of tracheostomy for ventilator liberation. Neurally-adjusted ventilatory assist (NAVA) is associated with improved patient-ventilator interaction compared to pressure support ventilation (PSV) because it reduces ineffective efforts and overassistance. NAVA may be an alternative for patients experiencing clinically important asynchrony on PSV by improving patient-ventilator synchrony.
What this paper contributes to our knowledge
We compared different patterns of asynchronies and respiratory mechanics between NAVA and variable PSV. In subjects with mild ARDS, NAVA and variable PSV had comparable effects on patient-ventilator asynchronies and pressure-time product. However, variable PSV reduced the variability of tidal volume and pressure support when compared with NAVA.
Methods
This was a randomized controlled study performed in the ICU of the University of Naples “Federico II.” The local ethics committee (Azienda Ospedaliero-Universitaria Policlinico di Federico II, Napoli. Ethic Committee, protocol number 132/17) approved the investigative protocol, and written informed consent was obtained from each patient or next of kin. A physician not involved in the study was always present for subject care. Our clinical trial was registered with ClinicalTrials.gov (NCT03018483). Inclusion criteria were age ≥ 18 y, endotracheal intubation, ventilation in controlled mode for at least 24 h consecutively and ready for assisted ventilation, PaO2/FIO2 200–300 and PEEP level < 10 cm H2O, hemodynamically stable, and without any neurological dysfunction/damage. Patients were excluded from the study if they were affected by neurological or neuromuscular pathology and/or known phrenic nerve dysfunction, presented any contraindication to the insertion of a nasogastric tube (for example, recent upper gastrointestinal surgery, esophageal varices), or denied informed consent.
After 24 h of VC-CMV with VT of 500 mL/min, PEEP of 6 cm H2O, and frequency of 15 breaths/min, subjects were randomized using a web-based encrypted platform with a 1:1 ratio randomization sequence. Subjects were assigned to one of the 2 groups: NAVA or variable PSV. Both assisted spontaneous ventilation modes were consecutively kept for 24 h unless clinical changes occurred as determined by the physician in charge. The assigned ventilation mode was applied immediately after randomization. The PEEP and FIO2 levels were left unchanged as those during controlled ventilation.
Subjects randomized in NAVA group were ventilated with a Servo-i ventilator (Maquet, Wayne, New Jersey) equipped with the NAVA software. After the randomization, the standard nasogastric tube was replaced with a 16 Fr, 125-cm EAdi catheter (Maquet). The EAdi catheter was first positioned according to the corrected nose-ear lobe-xyphoid distance formula.14 Subsequently, its position was titrated through the EAdi catheter position tool (Servo-i, NAVA software, Maquet).15 NAVA level was progressively titrated step by step by 0.2 cm H2O/μV to obtain a VT between 6–8 mL/kg. The EAdi trigger was set at a 0.5 μV threshold, whereas the NAVA inspiratory-to-expiratory cycling off is by default at 70% of the preceding EAdiPEAK. PSV was set as backup mode during NAVA. When backup ventilation was initiated, the operator reassessed the subject and their clinical status and, if stable, reinstituted NAVA ventilation.
Subjects randomized in variable PSV group were ventilated with Dräger Evita Infinity V500 ventilator (Dräger, Lübeck, Germany). Variable PSV generates random variation values in PS levels set by the operator, and those values will be applied to the PS delivered to the patient. The amount of variation desired can be adjusted from 0–100%. The maximum possible variation is limited by the set airway pressure (Paw) high-alarm threshold. PS level and variation around PS level were titrated to obtain a VT between 6–8 mL/kg predicted body weight. The PS variability was set at the highest level possible while not exceeding the maximal inspiratory pressure. The flow inspiratory trigger was set at 2 L/min, and expiratory trigger was set at 25% of the peak inspiratory flow.
During the study, the subjects received no sedatives or moderate doses of remifentanil and/or dexmedetomidine as clinically indicated. At the study inclusion, subjects’ demographic and medical characteristics, arterial blood gas analysis, Sequential Organ Failure Assessment score, and baseline ventilator settings were recorded.
A specific esophageal catheter equipped with esophageal balloon was inserted (Marquat, Boissy-Saint-Léger, France). During NAVA both catheters were used. The empty balloon catheter was advanced into the stomach, at which time the balloon was inflated, usually with 2.5 mL of air. The presence of a positive-pressure deflection during a spontaneous inspiration generally indicated that the balloon was in the stomach, provided that there was no diaphragmatic paralysis. Subsequently, the catheter was slowly withdrawn until a negative-pressure deflection replaced the positive deflection, indicating that the balloon was set in the lower third of the esophagus.16 The calibration procedure of esophageal pressure (Pes) consisted of an occlusion test (or Baydur maneuver) (2–5 inspiratory efforts).15 The proximal part of the catheter was connected to the pressure transducer (ICU-Lab software, KleisTEK, Bari, Italy). Flow was measured with a Fleisch pneumotachograph (Fleisch type 2, Vitalograph, Lenexa, Kansas) inserted between the Y-piece of the ventilator circuit and the endotracheal tube. The volume was obtained by electrical integration of the flow signal. Paw (located distal to the pneumotachograph) and Pes were measured with 2 differential pressure transducers (KT 100D-2, KleisTEK) (range ± 100 cm H2O). The Fleisch pneumotachograph and pressure transducers were connected to an ICU-Lab Pressure Box (KleisTEK) by 80-cm tube lines.
“Patient-ventilator asynchronies were evaluated in both NAVA and variable PSV according to Thille et al: (1) ineffective triggering (missed effort), (2) ineffective inspiratory triggering, (3) double-triggering, (4) auto-triggering, (5) prolonged cycle, (6) short cycle.17 Ineffective triggering was defined during VC-CMV and PSV as an abrupt Paw drop (≥ 0.5 cm H2O) simultaneous to a flow decrease (in absolute value) and not followed by an assisted cycle during the expiratory period. In PSV only, ineffective triggering could also happen during the inspiratory period but is related to a flow increase. Double-triggering was defined as 2 cycles separated by a very short expiratory time, defined as less than one-half of the mean inspiratory time, the first cycle being patient-triggered. Double-triggering occurs when the ventilator inspiratory time is shorter than the patient's inspiratory time. The patient's effort is not completed at the end of the first ventilator cycle and triggers a second ventilator cycle. Auto-triggering was defined as a cycle delivered by the ventilator without a prior Paw decrease, indicating that the ventilator delivered a breath that was not triggered by the patient. A prolonged cycle was defined as an inspiratory time greater than twice the mean inspiratory time. A short cycle was defined as an inspiratory time less than one-half the mean inspiratory time.”17
Flow asynchrony can be of two types: insufficient inspiratory flow and excessive inspiratory flow. In insufficient inspiratory flow, the flow received by the patient is lower than his/her ventilatory demand. Excessive flow asynchrony occurs because of an exaggerated delivery of inspiratory flow. The asynchrony index (AI) was calculated as follows: (total number of asynchronies/mechanical cycles plus missed efforts) *100. The AI was calculated at the beginning of NAVA or variable PSV and after 12 and 24 h of ventilation for 10 min.
Patient-ventilator asynchronies, breathing pattern, flow-time curve, VT (integration of flow-time curve), and work of breathing (integration of Pes-time curve) were calculated off line by the traces registered in the ICU-Lab (KleisTEK). We measured total PTP (PTPtot), minute PTP (PTPmin), PTP of the lung (PTPlung), PTP of the chest wall (PTPcw), and PTP over intrinsic PEEP (PTPPEEPi). The coefficient of variation, representing the breath-by-breath variability, was calculated by dividing the SD with the mean value of different variables like breathing frequency, VT, and PTP. The traces were recorded for 30 min at the beginning of assisted spontaneous ventilation, after 12 and 24 h. At the end of 30 min, 5 occlusion tests were recorded. The primary aim of this study was to evaluate the patient-AI between variable PSV and NAVA. Our secondary aims were to evaluate the coefficient of variation (CV) of breathing patterns and inspiratory effort between the groups.
Statistical Analysis and Sample Size
As no previous studies compared variable PSV and NAVA in human subjects, we calculated the sample size by including data on AI from the randomized controlled trial by Demoule et al.11 To reach a power of 80% with an alpha error 0.05, we had to include 13 subjects for each group. Data were analyzed by Shapiro test to investigate the normal distribution; parametric data were presented as mean and SD, nonparametric data as median and interquartile range. ANOVA was used for continuous variables, and proportions were compared with chi-square or Fisher exact test, as appropriate. P values < .05 were considered statistically significant. Analyses were performed with SPSS 20.0 (IBM, Armonk, New York).
Results
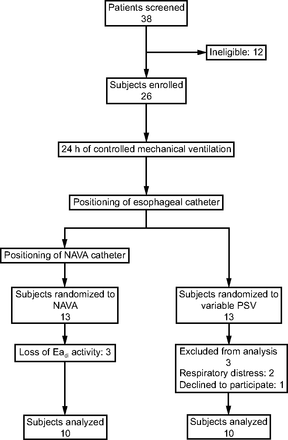
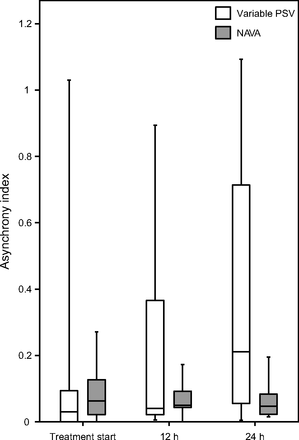
Of the 38 eligible patients, 26 were enrolled and randomized in the study after 24 h of VC-CMV machine-triggered and cycled. Thirteen subjects were randomized in NAVA group and 13 subjects in variable PSV group. In the NAVA group, 3 subjects were excluded because they lost the EAdi-pneumatic synchrony, whereas in variable PSV group 2 subjects dropped off because of respiratory distress that required a controlled ventilation period; one patient declined to participate. Figure 1 shows the flow diagram of enrolled subjects. The demographical characteristics of included subjects are shown in Table 1. There was no statistical difference in AI over time between NAVA and variable PSV groups (AI t0 P = .52, AI t12 P = .27, AI t24 P = .12) (Fig. 2). Ineffective trigger after 24 h of ventilation was higher in variable PSV than in NAVA (ineffective trigger after t12: variable PSV = 0.5, NAVA = 0; ineffective trigger after t24: variable PSV = 3.0, NAVA = 0, P = .03). Table 2 and supplementary Table 1S (See related supplementary materials at http://www.rc.rcjournal.com) report the statistical analysis for each type of asynchronies.
Flow chart. NAVA = neurally-adjusted ventilatory assist, EAdi = electrical activity of the diaphragm, PSV = pressure support ventilation.
Asynchronies index at t0, t12, and t24 between variable pressure support ventilation (PSV) and neurally-adjusted ventilatory assist (NAVA) groups. Asynchrony index (AI) (%) variable PSV t0 = 0.03 (0–0.09); AI (%) variable PSV t12 = 0.04 (0.02–0.36); AI (%) variable PSV t24 = 0.21 (0.05–0.71). AI (%) NAVA t0 = 0.06 (0.02–0.13); AI (%) NAVA t12 = 0.05 (0.04–0.09); AI (%) NAVA t24 = 0.05 (0.02–0.08). Data are shown as median and range interquartile in brackets. t0 = at the beginning of treatment, t12 = after 12 hours of treatment, t24 = after 24 hours of treatment. Y axis reports the AI as percentage.
Characteristics of Included Subjects
Patient-Ventilator Asynchronies During Neurally-Adjusted Ventilatory Assist and Variable Pressure Support Ventilation at Different Time Points
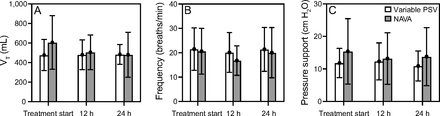
Coefficient of variation for VT was lower in variable PSV group over time compared with NAVA group (CV t0: P < .001; CV t12: P < .001; CV t24: P = .02) (Fig. 3) (Table 2S, see related supplementary materials at http://www.rc.rcjournal.com). Coefficient of variation for breathing frequency for variable PSV was higher over time (CV t0: P < .01; CV t12: P < .01; CV t24: P = .01) (Fig. 3) than NAVA. Coefficient of variation for PS was lower for variable PSV than NAVA over time (CV t0: P = .01; CV t12: P < .001; CV t24: P < .001) (Fig. 3) (Table 2S, see related supplementary materials at http://www.rc.rcjournal.com). PTPmin over time was not different between NAVA and variable PSV groups (PTPmin t0: variable PSV= 48.16 ± 50.5, NAVA 54.7 ± 46.9; P = .60. PTPmin t12: variable PSV= 55.4 ± 53.1, NAVA 71.5 ± 67.32; P = .57. PTPmin t24: variable PSV= 41.7 ± 36.9, NAVA 59.1 ± 67.8; P = .85) (Table 3S, see related supplementary materials at http://www.rc.rcjournal.com).
Tidal volume (VT), breathing frequency, and pressure support (PS) between variable pressure support ventilation (PSV) and neurally-adjusted ventilatory assist (NAVA) groups at different times. t0 = at the beginning of treatment, t12 = after 12 hours of treatment, t24 = after 24 hours of treatment.
Discussion
In this randomized controlled trial comparing NAVA with variable PSV after 24 h of VC-CMV in subjects with mild ARDS, we found no differences in asynchronies and in PTP as surrogate of work of breathing. To the best of our knowledge, variable PSV and NAVA have not been previously compared in critically ill mechanically ventilated subjects. To date, NAVA has only been compared with conventional PSV in clinical studies regarding asynchronies; however, a recent animal study reported no differences in asynchronies between NAVA and variable PSV.18 Asynchronies in critically ill patients may be associated with negative outcome. Thille et al17 showed that an AI > 10% was associated with an increase in the duration of mechanical ventilation and in the use of tracheostomy for ventilator liberation. NAVA was reported to be associated with an improved patient-ventilator interaction than PSV by reducing ineffective efforts and auto-triggering.19 These findings probably were due to the fact that NAVA trigger was directly adapted on the diaphragmatic activity, whereas PSV trigger was a pneumatic one.20 AI was higher in PSV compared with volume or pressure-controlled mechanical ventilation likely because of an inappropriate PS level set.21,22 In an experimental setting, double-triggered breaths did not differ between variable PSV and conventional PSV13; however, in subjects with mild to moderate ARDS, variable PSV was associated with less AI, double trigger, and double effort.14
In this study, we found no differences between NAVA and variable PSV in AI. We know from current literature that NAVA applied the level of PS proportionally to the inspiratory effort caught on the diaphragmatic electrical activity.14 On the other hand, during variable PSV, PS level was dissociated from the inspiratory effort and randomly varied according to a gaussian model.14 According to this, PS level in NAVA was intrinsically generated whereas in variable PSV was extrinsically generated.23 Varying methods of generating a PS level may affect VT or breathing frequency, but this evaluation is beyond the matter of this study. However, many physiological variables associated with breathing, such as VT or breathing frequency, exhibited significant breath-to-breath variability,14 and NAVA further increased variability of breathing frequency and VT.23 Whereas the PS level in NAVA depended on the patient’s intrinsic respiratory effort, in variable PSV it was completely independent of subjects and less influenced by respiratory center impairment, diaphragmatic dysfunction, or sedation.14 According to the intrinsic variability of breathing pattern, variable PSV appeared to provide more physiologic ventilation since each breath had different VT and each minute ventilation had different breathing frequencies and minute volumes.
In daily clinical practice, NAVA requires an experienced operator for the correct insertion of the esophageal catheter and the management of the EAdi pneumatic synchrony. Indeed, the loss of EAdi-pneumatic synchrony or an excessively low EAdi activity may be managed cautiously in the clinical context.19 On the other hand, variable PSV does not require an experienced operator nor any insertion of esophageal catheters, making it easier to use and more attractive than NAVA for patients and physicians.14
Unlike previous studies, we investigated the partitioning of respiratory mechanics in terms of PTPtot, PTPmin, PTPlung, PTPcw, and PTPPEEPi for variable PSV and NAVA. The PTP is measured as the time integral of the difference between the Pes tracing and the recoil pressure of the chest wall.23 PTP was developed to account for energy expenditures during the dynamic and isometric phases of respiration.23 The inspiratory PTP is a surrogate for work of breathing that correlates with the consumption of oxygen by respiratory muscles.24 PTP per breath and PTP per minute were significantly higher in NAVA than in conventional PSV, probably because subjects with the conventional PSV were over-assisted for most of the study period, whereas those with NAVA were properly or slightly under-assisted.19 PTP was not different between conventional and variable PSV in experimental and clinical studies.13,19 In the present study, we did not find any differences in PTPs between NAVA and variable PSV. Probably during variable PSV, the use of PS levels that randomly varied may avoid the over-assistance provided by a constant PS level.
This study has several limitations. First, this study had a limited cohort of subjects. Second, we only included subjects with a PaO2/FIO2 of 200–300, so our results should be used with caution when generalizing to other patient populations. Third, variable PSV was performed with the PS variability set as high as possible while not exceeding the maximal inspiratory pressure. Fourth, although we found different CV between the variable PSV and NAVA, this was not associated with clinical meaning.
Conclusions
In this randomized controlled trial including subjects with mild ARDS, NAVA and variable PSV had comparable effects on patient-ventilator synchronies and PTP. However, variable PSV reduced the variability of VT and PS when compared with NAVA. Further clinical studies should address the role and efficacy of NAVA and variable PSV both in moderate and severe ARDS.
Footnotes
- Correspondence: Maria Vargas MD, Department of Neurosciences, Reproductive and Odontostomatological Sciences, University of Naples “Federico II”, Via Pansini, 80100, Naples Italy. E-mail: vargas.maria82{at}gmail.com
See the Related Editorial on Page 620
Supplementary material related to this paper is available at http://rc.rcjournal.com.
This study was registered on ClinicalTrials.gov, number NCT03018483.
The authors have disclosed no conflicts of interest.
The study was performed at the University of Naples Federico II, Naples, Italy.
No funding was obtained for this study.
- Copyright © 2022 by Daedalus Enterprises