Abstract
BACKGROUND: During the coronavirus disease 2019 (COVID-19) pandemic, 60–80% of patients admitted to ICU require mechanical ventilation for respiratory distress. We aimed to compare the frequency of postextubation stridor (PES) and to explore risk factors in COVID-19 subjects compared to those without COVID-19.
METHODS: We performed an observational retrospective study on subjects admitted for severe COVID-19 requiring mechanical ventilation > 48 h during the first and second waves in 2020 and compared these subjects to historical controls without COVID-19 who received mechanical ventilation > 48 h between 2016–2019. The primary outcome was the frequency of PES, defined as audible stridor within 2 h following extubation.
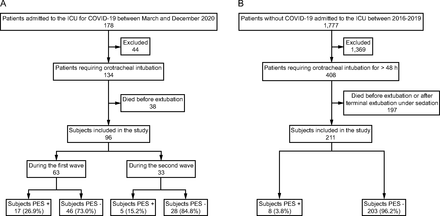
RESULTS: Of the 134 subjects admitted with severe COVID-19 requiring mechanical ventilation, 96 were extubated and included and compared to 211 controls. The frequency of PES was 22.9% in the COVID-19 subjects and 3.8% in the controls (P < .001). Factors independently associated with PES were having COVID-19 (odds ratio 3.72, [95% CI 1.24–12.14], P = .02), female sex (odds ratio 5.77 [95% CI 2.30–15.64], P < .001), and tube mobilization or re-intubation or prone positioning (odds ratio 3.01 [95% CI 1.04–9.44], P = .047) after adjustment on Simplified Acute Physiology Score II expanded). During the first wave, PES was significantly more common in subjects with a positive SARS-CoV-2 RT-PCR test on tracheal samples on the day of extubation (73.3% vs 24.3%, P = .018).
CONCLUSIONS: PES affected nearly one-quarter of subjects with COVID-19, a proportion significantly higher than that seen in controls. Independent risk factors for PES were COVID-19, female sex, and tube mobilization or re-intubation or prone positioning. PES was associated with persistent viral shedding at the time of extubation.
Introduction
Laryngeal complications are common in patients requiring invasive mechanical ventilation. Among them, postextubation laryngeal edema (PLE) usually manifests as stridor and can cause respiratory failure requiring re-intubation. PLE has been reported in 5.0–54.4% of patients and postextubation stridor (PES) in 13.0%.1,2 Absence of an air leak upon cuff deflation indicates a high risk of PLE but has shown poor performance in predicting PES.3 Independent risk factors for PLE/PES include female sex, large tube diameter, emergency intubation, and duration of mechanical ventilation.1 Knowledge of additional risk factors would help to select subjects for preventive treatment.
Laryngeal involvement may not be uncommon in COVID-19, as the disease affects the respiratory mucosa continuously from the nose to the lungs.4,5 Among patients hospitalized for COVID-19, 9–26% require ICU admission; and, among those, 60–80% require invasive ventilation.6 Intubation is often urgent and mechanical ventilation prolonged,7-9 predicting a high risk of PLE/PES.
A higher viral load is associated with greater severity of the lung inflammation.10,11 We are unaware of studies specifically addressing the frequency of PLE/PES in patients with COVID-19 and/or the possible role for the viral load as a risk factor for PLE/PES. In 2 case-series studies of ICU subjects with COVID-19, PLE/PES occurred in 2/8 subjects and 9/20 subjects, respectively.12,13 Given the scale of the current pandemic and uncertain pathogenesis of COVID-19, a thorough understanding of related symptoms is critical to facilitate their early diagnosis and appropriate treatment.
We hypothesized a high prevalence of PES in COVID-19 patients, due to possible virus-induced upper-airway mucosal inflammation at the time of extubation. Here, we report the prevalence of PES in a retrospective cohort of ICU subjects with severe COVID-19 compared to historical controls without COVID-19. Among the COVID-19 subjects and controls, we looked at the temporal association of PES with the viral load.
QUICK LOOK
Current Knowledge
Postextubation stridor (PES) has been reported in 13% of patients in ICU. Knowledge of additional risk factors would help to select patients for preventive treatment. Upper respiratory tract symptoms have been reported at the early phase of the COVID-19. We hypothesized a high prevalence of PES in patients with COVID-19 due to possible virus-induced upper-airway mucosal inflammation at the time of extubation.
What This Paper Contributes to Our Knowledge
Frequency of PES in subjects with severe COVID-19 was 6-fold higher than in general ICU subjects, and was associated with persistent viral shedding at the time of extubation. Routinely testing for the virus in tracheal samples before extubation might be a tool to identify subjects at high risk for PES.
Methods
Ethics
Anonymized data were collected after ethical approval (Ethical Committee CESRLF 20–42) provided by ethics committee of the French Intensive Care Society, Paris, France, on May 8, 2020. If the subjects were unable to provide informed consent at inclusion, investigators sought consent for participation from surrogates. If the surrogates chose not to give consent, the patients were not included. Written consent was then requested from all included subjects as soon as they regained competence. For the historical cohort, all subjects received an informative letter. The trial was investigator-initiated and received no industry support.
Study Population
We included consecutive subjects admitted between March–June 2020 (first COVID-19 wave in France) and between July–December 2020 (second COVID-19 wave) to the medical-surgical ICU of the Versailles Hospital (France) with reverse-transcriptase polymerase chain reaction (RT-PCR) assays of nasopharyngeal or tracheal samples positive for SARS-CoV-2 and a requirement for mechanical ventilation. We compared these subjects to historical controls who were admitted consecutively to the same ICU between 2016–2019 without COVID-19 and who required mechanical ventilation for > 48 h. We excluded patients who died before extubation.
Study Outcome
The primary outcome was a stridor audible to the naked ear within 2 h after extubation and requiring a curative medical treatment.
Study Setting and ICU Management
During the first wave, the 28 beds in our ICU were managed by intensivists, and 15 beds created in operating rooms were managed by anesthesiologists with the same COVID care protocol. All 43 beds were occupied by patients with COVID-19. During the second wave, 22 ICU beds were reserved for COVID-19 subjects. The management of ARDS complicating COVID-19 followed the French Intensive Care Society guidelines.14 During the first wave, specific therapeutic interventions such as rescue corticosteroid therapy were discussed by the medical team for patients with moderate-to-severe ARDS whose PaO2/FIO2 was below 150 despite neuromuscular-blocking agents and prone positioning and whose pulmonary compliance was below 30 mL/cm H2O. When deemed indicated, we gave intravenous dexamethasone, 20 mg once daily from day 1 to day 5, then 10 mg once daily from day 6 to day 10.15 During the second wave, all patients with severe COVID-19 received 6 mg of dexamethasone per d for 10 d.16 All intubations were performed by experienced prehospital emergency physicians or intensivists. The use of videolaryngoscopy with a hyper-angulated stylet or bougie and the tracheal tube diameter was at the discretion of the intubating physician; however, the maximum tube size was 8, with subglottic suction. During the ICU stay, cuff pressures were routinely checked every 4 h and maintained between 25–30 cm H2O. Extubation was decided by the bedside physician according to current guidelines17 after a successful 1-h spontaneous breathing trial with minimal pressure support and no PEEP.18 The decision to perform a cuff leak test was at the discretion of the physician. A positive cuff leak test was defined as < 110 mL of leakage after tube balloon deflation just before extubation.18,19 PES prevention was at the discretion of the bedside physician; when performed, it consisted in preextubation intravenous methylpredni-solone, 80 mg over 12 h.20 The nasogastric tube was removed and the trachea and mouth were carefully suctioned. The cuff was then deflated and the trachea suctioned again. Treatment for PES consisted of nebulized epinephrine with or without intravenous steroids, depending on the decision of the medical team. Extubation failure was defined as the need to re-intubate within 48 h after extubation, irrespective of the reason.
Data Collection
Data were collected from the electronic medical records using a standardized data collection form. PES occurrence was searched directly in nurses, physiotherapists, and medical records or indirectly through prescribed treatments such as postextubation systemic steroids and epinephrin or steroid nebulization. For each subject, we recorded age, sex, body mass index, medical history, clinical signs, microbiological findings, characteristics of intubation (prehospital or in the ICU, intubation difficulties), severity score assessed with Simplified Acute Physiology Score II expanded (SAPS II), mechanical ventilation duration (from intubation to first planned extubation), and prolonged intubation, defined as ≥ 7 d of invasive ventilation. In subjects with respiratory distress requiring immediate re-intubation but without PES, mechanical ventilation duration was counted from the first intubation to the second extubation. We also recorded endotracheal tube (ETT) diameter and the ratio of the subject’s height to the tracheal tube diameter (in millimeters), whether accidental extubation occurred, the number of intubations during the ICU stay before the last extubation, and the reasons for re-intubation (extubation failure or tube obstruction). Difficult intubation was defined as intubation after > 2 laryngoscopies performed by a trained physician with a Cormack-Lehane grade 3 or 4 or a need for rescue oxygenation.21 We recorded whether subjects received steroids before extubation and specially within the 48 h before extubation and the reason for steroid administration (septic shock, persistent severe ARDS, bronchospasm, or preventive or curative treatment of PES). Finally, we collected the results of RT-PCR assays for SARS-CoV-2 on tracheal samples done each week and within 48 h before extubation. A positive RT-PCR for SARS-CoV-2 was expressed in cycle thresholds. The cycle threshold value was the number of cycles it takes for the PCR test to detect the virus; the threshold was 40 cycles. If the virus was found in a low number of cycle thresholds, it meant that the sample contained a large amount of virus, thus a higher viral load.
Statistical Analysis
Quantitative parameters were described as median (interquartile range [IQR]) and qualitative parameters as number (percentage). We compared categorical variables using Fisher exact tests and continuous variables using Wilcoxon rank-sum tests. All tests were 2 sided, and P values < .05 were considered significant.
To assess risk factors for PES, we compared subjects with versus without PES by building a multivariate model using variables associated with P values < .15 by univariate analysis and variables deemed clinically relevant. We adjust our multivariate model on severity based on SAPS II. All continuous variables were checked for log-linearity. Non-log-linear variables were transformed into dummy variables using the median value as the cutoff. The following variables were included into the multivariate model: female sex, body mass index, difficult intubation, COVID-19 status, tube mobilization or re-intubation or prone positioning, prolonged mechanical ventilation, and subject height/tube diameter. We used a backward stepwise multivariate approach, and we chose the final model according to the best Hosmer-Lemeshow goodness-of-fit test and area under the receiver operating characteristic curve estimated by the C statistic. Associations of factors with PES were reported as odds ratios with their 95% CIs.
To test the robustness of the comparison of the frequency of PES in the COVID-19 subjects and controls, we conducted an exploratory analysis using a propensity score based on 1:1 exact matching on each score variable, with no replacement, using a prespecified caliper width of 0.2. This matching process based on propensity score was used to equalize potential risk factors of PES in both groups. The variables included in the propensity score were demographic and baseline characteristics associated with PES with P < .05 by multivariate logistic regression and data about prolonged mechanical ventilation. These variables were COVID-19 status, female sex, and tube mobilization or re-intubation or prone positioning. Each of 65 COVID-19 subjects was matched on the propensity score to a control.
Finally, we sought to identify COVID-19-specific risk factors for PES during the first wave to avoid a potential effect of routine corticosteroid therapy. Bonferroni correction was applied to adjust for multiple comparisons. We also compared the main characteristics of the subjects between the first and second COVID-19 waves. All analyses were performed using R version 4.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Figure 1 is the subject flow chart. Of the 134 subjects admitted with severe COVID-19 and intubated during the first and second waves, 38 died before extubation, leaving 96/134 (71.6%) COVID-19 subjects for our study. We screened 408 consecutive controls, among whom 197 died before extubation, leaving 211/408 (51.7%) controls for our study. Table 1 reports the main characteristics of the COVID-19 subjects and controls.
Flow charts. A: COVID-19 subjects, B: Control group.
Main Features of the Overall Population, Subjects With COVID-19, and Controls Without COVID-19
Frequency of PES and Associated Factors by Multivariate Analysis (Table 2)
PES occurred in 22.9% of the COVID-19 subjects and 3.8% of the controls (P < .001), leading to re-intubation in 36% versus 25%, respectively, (P = .68). By multivariate analysis adjusted on SAPS II, 3 factors were significantly associated with a higher prevalence of PES: COVID-19 (odds ratio 3.72 [95% CI 1.24–12.14], P = .02), female sex (odds ratio 5.77 [95% CI 2.30–15.64], P < .001), and tube mobilization or re-intubation or prone positioning (odds ratio 3.01 [95% CI 1.04–9.44], P = .047). Prolonged mechanical ventilation was not associated with PES after adjustment.
Risk Factors for Postextubation Stridor by Univariate and Multivariate Analysis
The sensitivity analysis comparing 65 COVID-19 subjects to 65 propensity score-matched controls produced comparable results, with a significantly higher frequency of PES in the COVID-19 subjects (19/65, 29.2% vs 3/65, 4.6% in the controls; P < .001) (eTable 1, see related supplementary materials at http://www.rc.rcjournal.com).
Specific Risk Factors for PES in the First Wave Subjects, by Univariate Analysis (Table 3)
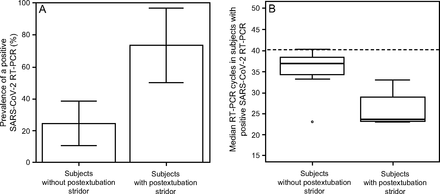
Subjects with PES had significantly shorter mechanical ventilation duration, 11 (8–18) d versus 25 (13–39) d for subjects without PES, P = .02. PES was significantly more common in subjects with a positive SARS-CoV-2 RT-PCR test on tracheal samples on the day of extubation (73.3 vs 24.3%, P = .018) (Fig. 2). Furthermore, RT-PCR cycles on the day of extubation in subjects with PES were 23.8 (23.16–29.00) cycle thresholds versus 37 (34.72–38.22) cycle thresholds for subjects without PES. This difference was not statistically significant, after Bonferroni correction, P = .13.
Risk Factors for Postextubation Stridor in Subjects During the First COVID-19 Wave
A: Prevalence of positive SARS-CoV-2 reverse-transcriptase-polymerase chain reaction (RT-PCR) tests on tracheal samples on the day of extubation during the first wave of COVID-19 in subjects with and without postextubation stridor. The boxes indicate the percentage, and the lower and upper brackets the 95% CI. P = .02 (Bonferroni correction). B: Box plot of scores for median SARS-CoV-2 RT-PCR cycles during the first wave of COVID-19. Boxes show the middle 50% of the values, and the lower and upper ends of the box the 25th and 75th percentiles, respectively. Horizontal lines indicate the median, and the exterior point denotes outlier. The dashed horizontal line indicates the maximal cycle threshold in case of positive PCR. P =.13 (Bonferroni correction).
Compared to the first COVID-19 wave, the second wave was characterized by comparable RT-PCR cycle thresholds in subjects with positive SARS-CoV-2 RT-PCR results in tracheal samples on the day of extubation (P > .99) and by a significantly higher proportion of subjects receiving intravenous steroids before extubation (P < .001). The prevalence of PES was not significantly different during the second wave (P > .99) (eTable 2, see related supplementary materials at http://www.rc.rcjournal.com).
Discussion
Our study provides the first evidence that COVID-19 is an independent risk factor for PES. The frequency of PES was 6-fold higher in COVID-19 subjects than in controls. Other independent and significant risk factors for PES adjusted on severity were female sex and tube mobilization or re-intubation or prone positioning. Among the subjects of first COVID-19 wave, PES was associated with persistent pulmonary viral shedding by univariate analysis.
PLE is a common cause of airway obstruction after extubation in ICU patients and is usually attributed to direct mechanical trauma to the larynx by the ETT. Previously identified risk factors are female sex, large tube diameter, high cuff pressure, emergency intubation, and difficult intubation. Whether a longer mechanical ventilation duration increases the risk is debated.20 In our study, independent factors associated with a higher risk of PES were female sex, tube mobilization or re-intubation or prone positioning, and having COVID-19. In 2 small case-series studies, the frequency of PLE/PES in subjects with COVID-19 ranged from 25–45%.12,13 Of note, we recorded PES but did not perform routine laryngoscopy to identify PLE without stridor.
A sentivity analysis from the first wave, during which steroid therapy was not given routinely to subjects requiring mechanical ventilation for severe COVID-19, showed that 2 factors were significantly associated with PES by univariate analysis, namely a shorter mechanical ventilation duration and a positive SARS-CoV-2 RT-PCR test on a tracheal aspirate sample taken on the day of extubation. Furthermore, the risk increased with the viral load in the tracheal sample. Thus, persistent pulmonary SARS-CoV-2 shedding may contribute to the development of PES. A case-control study showed that 14 of 30 subjects with COVID-19 who received mechanical ventilation for > 2 weeks had full-thickness tracheal lesions or tracheoesophageal fistulas compared to 1 of 45 controls without COVID-19.22 SARS-CoV-2 penetrates into the cells after binding to the angiotensin-converting enzyme 2 (ACE2) receptor present on the surface of several human cell types, including respiratory-tract epithelial and endothelial cells.23 Viral injury induces localized microvascular inflammation, which triggers endothelial activation, leading to vasodilation.24 In the early phases of the infection, ACE2 consumption by viral entry is expected to increase local angiotensin II concentrations, inducing vasoconstriction and stimulating inflammation. This initial increase in local angiotensin II is followed by a gradual decrease down to values well below physiologic levels, resulting in vasodilation, increased capillary leakage, and impaired endothelial conductance and autoregulation.
Althrough prolonged mechanical ventilation is usually reported as a risk factor for PLE/PES, the association was not significant in multivariate analysis. Moreover, during the first COVID-19 wave, subjects with PES had shorter mechanical ventilation duration. A shorter ventilation duration may correlate with a higher probability of the tracheal samples still being positive. Thus, the relevant association here may be between PES and persistence of the virus. In a placebo-controlled trial of dexamethasone for preventing PLE, by univariate analysis, a shorter time on mechanical ventilation was also associated with PLE.20 Also, during the first wave, the subjects with the longest mechanical ventilation duration were those with the most severe disease and, therefore, more often received rescue corticosteroid therapy, which may have decreased their risk of PES. Thus, of the 13 subjects in our study who required rescue corticosteroid therapy, 12 had no PES. Several studies found that preventive intravenous methylprednisolone before extubation decreased the risk of PLE.20,25 Our protocol for rescue corticosteroid therapy during the first wave was a total of 150 mg of dexamethasone (equivalent of 800 mg of methylprednisolone),15,26 whereas the protocol used for PES prevention was 80 mg of methylprednisolone.20 During the second wave, subjects with severe COVID-19 routinely received a total of 320 mg of methylprednisolone.16,27 Thus, steroid use was more common during the second wave, yet neither the frequency of PES nor the result of SARS-CoV-2 RT-PCR tests on the day of extubation was significantly different between these 2 periods.
Huge efforts are being made to better understand and treat severe COVID-19 and, more specifically, to prevent progression to severe disease. As experience accumulates and new treatment protocols are identified, the duration of mechanical ventilation may become shorter, leading to a larger proportion of patients being extubated with positive SARS-CoV2 RT-PCR tests and, therefore, possibly with a greater risk of PES.28 Several studies in general ICU populations have shown that a positive cuff leak test combined with the presence of risk factors can identify patients at increased risk for PLE.29 We have no data on the exact mechanism of PES in our study, although the association with persistent viral shedding suggests a role for persistent SARS-CoV-2-driven inflammation of the larynx. Performing RT-PCR tests just before extubation and considering preventive treatment of PES in the event of positive results is a strategy that may deserve evaluation.
We must acknowledge several limitations. First, methodological limitations are inherent in the retrospective data collection and observational design. In order to consider these information biases, the study period was restricted to 5 years. We also performed a propensity score to adjust for confounders, and results were consistent. Second, our outcome was clinical as we considered only PES; we cannot exclude a variability of clinical diagnosis among clinicians according to the sound of stridor and according to the COVID-19 status. Therefore, we probably underestimated the true prevalence of PLE; however, only clinical PLE is relevant to patient management, and visual confirmation of laryngeal edema may result in aerosolization of virus-containing droplets. Third, we did not perform a routine and standardized assessment of the cuff leak test, and steroid therapy to prevent PLE/PES was at the discretion of the medical team with a joint protocol between intensivists and anesthesiologists during the COVID waves. Fourth, controls differed from the COVID-19 group according to the reason of intubation, but this seems not to be a risk factor for PES in the literature. Fifth, not all subjects underwent RT-PCR tests on tracheal samples just before extubation. Sixth, during the first wave, rescue dexamethasone therapy was not systematically given Finally, risk factors for PES among COVID-19 subjects were sought only by univariate analysis. Overall, our results can only be interpreted as preliminary and hypothesis- generating. Further studies are needed to better understand the risk of PLE/PES in patients with severe COVID-19 disease and to evaluate preventive strategies.
Conclusions
COVID-19 was an independent risk factor for PES in a large cohort of consecutive subjects compared to individually matched controls. PES occurred in 23% of subjects with COVID-19 and was associated with persistent viral shedding at the time of extubation. Testing for the virus in tracheal samples before extubation might identify patients at high risk for PES, and preventive steroid therapy could be discussed. Further studies are warranted to evaluate this strategy.
Acknowledgments
The authors thank the Centre Hospitalier de Versailles for editorial assistance.
Footnotes
- Correspondence: Marine Paul MD, Intensive Care Department, Centre Hospitalier de Versailles - Site Andre Mignot, 177 Rue de Versailles, 78150 Le Chesnay, France. E-mail: mpaul{at}ch-versailles.fr
See the Related Editorial on Page 772
Supplementary material related to this paper is available at http://rc.rcjournal.com.
The authors have disclosed no conflicts of interest.
- Copyright © 2022 by Daedalus Enterprises