Abstract
Electrical impedance tomography is no longer a new technology, but its clinical use at the bedside is still in its primary stage. Global research has drastically increased since its commercial availability, and this has slowly begun to make its way into routine clinical bedside use in some areas of the world. This paper will provide the bedside clinician an introduction to the technology, how it is used, and the most common applications found in the literature.
Introduction
Bedside imaging of the respiratory system has long been considered an essential part of ICU patient assessment. However, the use of traditional bedside imaging techniques as a monitoring tool is either not possible or challenging. Chest x-rays are static images that do not demonstrate changes that occur during breath cycles (inspiration and exhalation). Lung ultrasound can visualize dynamic changes during breath cycles but is limited by being able to visualize one area under the probe and cannot provide a real-time image of all lung regions at the same time.
Electrical impedance tomography (EIT) is a radiation-free imaging technique that provides functional lung information across multiple thoracic regions at once, allowing specialized monitoring during breath cycles. Although EIT is not considered a diagnostic tool like computed tomography (CT) or lung ultrasound, it can facilitate early recognition of certain serious events such as main stem intubation, development of a pneumothorax, and pleural effusions; these conditions would then require follow-up diagnostic interventions for confirmation. Where EIT has become useful at the bedside is the monitoring of changes that occur in the distribution of ventilation across the thorax while an intervention is taking place such as assessing the response to increasing PEEP and the ability to set a PEEP level that aims to balance regional overdistension and collapse. The commercial availability of EIT devices globally has resulted in a considerable increase in published research highlighting the clinical applications and potential benefits of monitoring the regional distribution of ventilation at the bedside.
Basic Principles of EIT
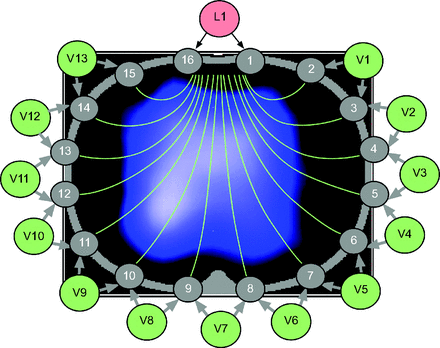
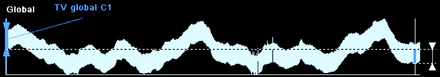
EIT uses a series of 16 or 32 electrodes (depending on the device) placed around the thorax between the fourth and fifth intercostal space. The electrodes are integrated into a rubber belt, or adhesive strips, of various sizes. A master cable connects the belt (or adhesive strips) to the device itself. A small electrical current (not felt by the patient) is sent from a starting lead to each lead in series around the chest. Adjacent pairs of electrodes measure the impedance (resistance to the current) compared to the starting lead, and an image is constructed that reflects the measured impedance (Fig. 1). The starting lead shifts to the next chest lead, and the process is repeated, creating additional images. A full cycle around the chest will generate a tomographic slice of the region, a caudal-to-cranial view of the chest like a CT scan. Up to 50 cycles per second are performed by the device. A reference lead is placed on tissue outside the thoracic space; this reference ensures that when the EIT images are reconstructed they all compare impedance changes to a common tissue reference point that does not include air. The reconstructed images are processed and displayed on the screen in real time as a dynamic (changing) image that represents the distribution of ventilation across the measured field.
Using a 16-lead electrical impedance tomography (EIT) belt, an alternating current is delivered from the first lead pair (L1) and sent to 13 other pairs of electrodes (V1–V13). L1 is then shifted to the next pair and sent to the next 13 pairs of electrodes. L1 moves around the chest, and after one full rotation, there are 16 profiles, each with 13 paired voltage measurements. This results in a frame that consists of 208 values that are then reconstructed into a single cross-sectional image. EIT devices repeat this up to 50 cycles per second to provide a dynamic image projected onto the screen of ventilation occurring through the lung.
The Importance of Belt Placement
Belt placement is important to optimize the relationship between the change in impedance and tidal volume (VT) distributed through the lung.1 The tidal impedance change (maximum impedance − minimum impedance) is referred to as tidal impedance variation (TIV), and proper belt placement allows the ratio between TIV and VT to be consistent. The consistency of the TIV/VT relationship is important for the accuracy of the EIT measurements discussed later. The electrode belt placement requires direct contact with skin. Therefore, surgical site bandages or damaged tissue (ie, burns) must be avoided. Additionally, the length of time the belt is applied may vary based on manufacturer; these time limits of application are normally based on the avoidance of skin breakdown. Patients with a significant amount of body hair may have issues with obtaining an optimal connection. In this circumstance, electrode contact gel (eg, ultrasound gel) can be used to improve contact between the tissue and belt.
The presence of an active pacemaker is also considered a contraindication to using EIT. The electrical signal used by EIT is not felt by the patient. However, electrical interference between the device and the pacemaker might occur and should, therefore, be avoided. Additionally, contact with any electrode cable should be avoided as it can interfere with the signal.
EIT Resolution: Spatial and Temporal
Spatial resolution refers to the lung area or region represented by the image pixels. The EIT belt is normally < 4 cm in width, but the cross section of the belt represents an area of approximately 10 cm of the lung in the caudal-cranial direction. Because the belt represents a large area rather than a more precise area (ie, lobar segment or alveoli), the spatial resolution is considered low, unlike CT or magnetic resonance imaging (MRI) that has high spatial resolution useful for diagnostic purposes (eg, lung disease).
Temporal resolution is related to frequency of obtaining images (cycles around the chest per second). EIT devices provide up to 50 cycles of image capture, making them higher than CT and MRI for detecting dynamic movement of ventilation throughout the thoracic space being measured.
Distribution of Ventilation
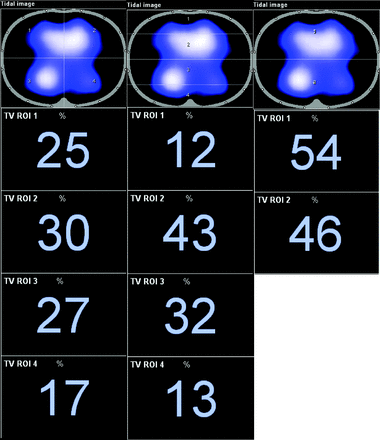
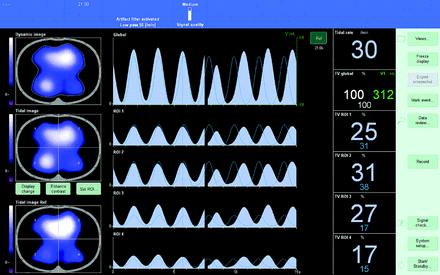
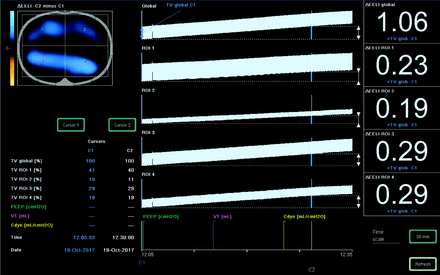
Distribution of ventilation measured by EIT was validated against distribution measured with CT by Victorino et al in 2004.2 They concluded that changes in air content measured with CT predicted regional changes in impedance (R2 = 0.93), which means that impedance changes measured with EIT explain ventilation changes observed in CT (93% of its variance).2 In addition to simply visualizing the distribution of ventilation throughout the lung, a variety of measurements can be obtained using the distribution of ventilation data. Regions of interest (ROIs) are selected to isolate and compare distribution between the different regions of the lungs. These regions separate the thoracic image into quadrants, layers, or ventral/dorsal regions; and during inspiration, the distribution of ventilation is visualized on the device monitor and displayed as a percentage of the total amount of volume (represented by the tidal variation) delivered to each ROI (Fig. 2). Not only is there a visual image reconstructed to show where distribution occurs through the lung, there are waveform graphics of impedance that represent the VT being delivered to each region (see Fig. 3). With this information, several measurements obtained using EIT can be made; a list of the most common measurements is presented in Table 1 and will be discussed further.
Different regions of interest (ROIs) utilized by electrical impedance tomography. Left image is separated into quadrants; the middle image is layers; the right image is ventral/dorsal. The values below each image are the percentage values of the entire image distributed to each ROI.
The main display of a PulmoVista 500 device. The images on the left are also represented by waveform graphs in the center and percentage distribution on the right. The waveforms and percentage distribution are separate and represent the global and regional information. ROI = region of interest.
Electrical Impedance Tomography Measurements Commonly Used
EIT Measurements
There are many different EIT measures that can be used to assess the changes in lung function after clinical interventions (ie, starting a therapy, making ventilator changes). The following are the most-used measurements and a description of the information they provide arranged in order of complexity (starting with the simplest measurement); it is not a complete list.
Tidal Impedance Variation
TIV is simply the change in impedance from end exhalation to end inspiration; it represents the change in VT being distributed through the lung. It is visually represented as an impedance waveform on the EIT device, like that of a VT waveform on a ventilator (see Fig. 3).
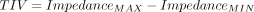
Dorsal Fraction of Ventilation
A simple concept of measuring the predominant area of ventilation distribution is observing the dorsal fraction of ventilation (DFV), which describes the percentage of ventilation reaching the dorsal lung regions and can be used to determine if an increase in PEEP should be attempted and also as an indication to decrease PEEP3 (described later).
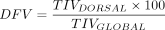
Regional Compliance
When EIT devices calculate compliance of various regions, TIV is used as the numerator (like VT) for a compliance measurement with the change in pressure as the denominator. The compliance can either be measured globally (all areas of the lung) or regionally by setting the desired ROI to be assessed. The compliance measurements are also used in an analysis to compare the changes in compliance that occur in different lung regions during a decremental PEEP titration, indicating areas of potential overdistension and collapse (described later).
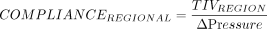
Intratidal Gas Distribution
The intratidal gas distribution (ITV) separates a single breath into 8 different time segments as a percentage of an entire breath. ITV compares the distribution of ventilation in the ventral and dorsal ROI at each time segment.4 This allows a more in-depth analysis of changes that occur throughout inspiration. The utility of this single breath analysis is to visually assess the presence of tidal recruitment (opening from a state of atelectasis) as well as overdistension. It has been used as a method for determining optimal PEEP.5 It should be noted that commercially available devices do not analyze ITV at the bedside; it requires an off-line analysis.
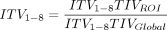
Additionally, the ITV of the ventral regions can be divided by the ITV of the dorsal lung regions to provide the ITV index.5
Regional Ventilation Delay
Regional ventilation delay describes regions of the lungs where impedance changes later than the global impedance change (late opening of lung units). The late opening may be due to regions of atelectasis opening during tidal ventilation; it can also be present when reverse triggering is present (patient effort occurs after the onset of a ventilator-delivered breath, causing a change in the direction of ventilation distribution), and it can also appear when there is excessive effort resulting in the pendelluft effect occurring between different regions of the lungs.6
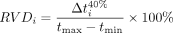
(i = each pixel; Δti40% = the time from the start of global impedance change to reach 40% of the maximum impedance change; tmax − tmin = inspiration time.)
Global Inhomogeneity Index
The global inhomogeneity index (GI) is one of the more complex analyses performed (off line) by EIT devices. It is a measure of how homogenous ventilation distribution is throughout the lung. Lower values indicate a more homogenous distribution, whereas higher values indicate increased inhomogeneous distribution. The measurement is done after PEEP titration, and the value at each PEEP step is compared; the PEEP level with lowest GI index is considered the preferred or optimal setting.7
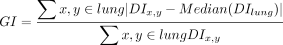
(DI = impedance variation; xy = a specific lung area pixel; lung = all pixels of the lung area)
Clinical Application of EIT
Assessing the Response to PEEP
The ability of EIT to monitor both global and regional distribution of ventilation gives it a unique advantage in the assessment of PEEP responsiveness and alveolar recruitment. When distribution of ventilation occurs predominantly in the ventral regions, the goal of increasing PEEP would be to improve distribution of ventilation to the dorsal lung regions, resulting in a more homogenous distribution of ventilation. This can be visually assessed at the bedside using simple assessments such as the DFV described earlier, where ∼45–50% implies a more balanced distribution of ventilation between the ventral and dorsal lung regions.3 A number of the measurements described earlier can be used to assess responsiveness to PEEP and will be described further.
Optimal PEEP Using Changes in Regional Compliance
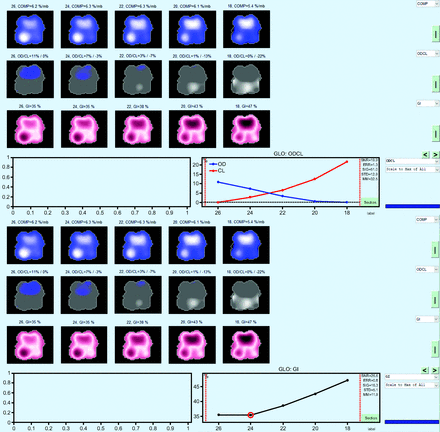
Whereas improvements to regional ventilation or increased homogeneity can indicate recruitment, it does not directly assess the possibility of overdistension resulting from recruitment. All EIT devices have software capable of comparing overdistension resulting from high PEEP to collapse resulting from low PEEP. This analysis is made following a decremental PEEP titration. The decremental PEEP trial begins from a PEEP level considered to be higher than what would be required for the patient, and PEEP is decreased in increments of 2 cm H2O to a minimally acceptable PEEP level. The resulting analysis will plot the percent of compliance lost at the highest PEEP (risk of overdistension) compared to the lowest PEEP level, and on the same graph it will plot the percent of compliance lost at the lowest PEEP (risk of collapse) compared to the highest PEEP level (Figure 4).8 Different approaches have been used to set PEEP using this analysis. These include (1) setting PEEP where there is < 10% collapse; (2) setting PEEP where the 2 lines intersect; or (3) if the 2 lines intersect between 2 different PEEP levels, the PEEP where the GI index is the lowest is used. In Figure 4, these approaches would result in a PEEP selected between 22–24 cm H2O in a patient who was very responsive to higher levels of PEEP. The currently available data do not provide evidence of superiority for one technique over another. However, the ability to assess risk and benefits of PEEP through regional compliance changes is a method of individualizing PEEP gaining popularity and study. The challenge for clinical trials using EIT-selected PEEP will be how the device is used and how often. Considering how dynamic patients can be early in the course of mechanical ventilation, if EIT is only used once to set PEEP without follow-up use it does not answer the question of whether using EIT to guide PEEP is useful.
Example of the overdistention/collapse (OD/CL) analysis done after a decremental PEEP trial and the global inhomogeneity index (GI) analysis for the same decremental PEEP trial.
Optimal PEEP Using ΔEELI
When CPAP or PEEP is applied or increased, there is always an increase in end-expiratory lung volume (EELV). The additional pressure might increase EELV in the areas currently being ventilated or recruit areas that were previously collapsed, something referred to as recruited volume. Bedside methods for assessing recruitment volume are multiple pressure volume curves (flow-derived method), the helium dilution technique, nitrogen washin/washout, and the recruitment to inflation ratio.9-11 EIT uses the change in end-expiratory lung impedance (ΔEELI) to estimate the changes in EELV. Recruited volume estimated by EIT is significantly correlated with the nitrogen washin/washout method and with the flow-derived method; however, the limits of agreement are highly variable.12-14 Regardless, the additional information provided by EIT in the impact of this recruited volume is the improvements in regional compliance or any loss of regional compliance that could indicate hyperinflation. This allows the assessment of the potential risk and benefit of increasing PEEP as described in the previous section.
EIT as an Incentive to Decrease PEEP
Whereas many studies have assessed methods of individualizing PEEP, there is a lack of studies that has focused on optimal methods to determine when to decrease PEEP. Methods to individualize PEEP may involve setting PEEP, weaning FIO2 as tolerated, then a slow reduction in PEEP at various time intervals (ie, every 6 h). When EIT is used to assess recruitment, or during a decremental PEEP trial, it is often noted that distribution to the nondependent (ventral regions) while supine is diminished when PEEP is high, resulting in a DFV to be increased > 50%. When applying EIT at the bedside, this increased DFV is easily recognizable and can indicate the need to perform a stepwise decrease in PEEP to achieve a more even distribution of ventilation between the ventral and dorsal regions (ie, DFV ∼45–50%).3
Other Clinical Applications
Distribution Changes During Spontaneous Breathing Trials
The ability for EIT to monitor distribution of ventilation changes that occur during mode changes makes it an interesting tool for the assessment of weaning readiness or as a predictor of spontaneous breath trial (SBT) success. Whereas EIT has been used in studies assessing its potential role for this, the studies include varying methods of performing SBTs and different types of patient populations and, therefore, may be difficult to generalize the findings to all patients at this time.15-18 Additionally, the measurement that provides the best information remains unclear and likely may depend on which method of SBT is used.
Pendelluft Resulting From Excessive Effort
The idea that mechanically ventilated patients with excessive effort can worsen or even cause lung injury is a concept referred to as patient self-inflicted lung injury. Whereas there is a strong physiological rationale for its existence, it is uncertain at this time if the efforts to prevent it (sedation, paralysis) would be more injurious than allowing excessive effort. Additionally, the bedside methods for determining excessive effort may not correctly identify those with the highest risk of patient self-inflicted lung injury. The use of EIT to determine the potential negative effects of excessive effort has been published.6,19 The concept that localized strain can occur as patients draw volume not only from the ventilator but also from other areas of the lungs through the pendelluft effect. It's possible that excessive effort in a lung with homogeneous distribution of ventilation is less injurious than excessive effort resulting in pendelluft and localized strain within certain areas of a heterogeneous lung.
Perfusion
Similar to measuring the impedance of air across the thorax, EIT devices have the ability to measure the impedance of fluid to determine distribution of perfusion. This is normally done through a brief interruption in ventilation (apnea period is required) while fluid (typically hypertonic saline) is injected, and the impedance changes during this time are captured, and distribution of these impedance changes due to fluid is displayed on the screen. Additional software can provide a V̇/Q̇ analysis by overlapping distribution of ventilation and perfusion.20,21 The ability to monitor perfusion is not universally available on all EIT devices at this time but likely will be. The titration of PEEP always has potential to impact perfusion through the lung, and the ability to assess changes due to changes in PEEP is a welcome addition.
Limitations of EIT
As previously mentioned, proper belt placement is important, and there are situations in which the belt cannot be applied, and therefore, this is a limitation of EIT technology. The presence of an active pacemaker was also mentioned and is a contraindication to the application of EIT electrodes.
Measurement Artifacts
Whereas many devices measure the impedance change from a baseline value, the baseline can be affected by movement, external pressure applied to the leads, and rapid changes in the patient’s fluid status. An active patient who is moving or repositioning themselves will greatly impact the baseline trend in EELI. However, patients who are completely passive can also have artifacts from pressure applied to electrodes. An example of this artifact is shown in Figure 522 where the ICU bed is providing percussion that has an impact on the baseline (EELI) trend. Rapid changes in fluid status can impact the tissue reference that can cause a shift in the EELI baseline downward when fluid boluses are given or upward during aggressive diuresis (Fig. 6).22 It is worth noting that EIT measurements that assess regional compliance or distribution of ventilation are not impacted by these artifacts; only the assessments using the trends of baseline (ie, ΔEELI) are affected by these artifacts.
The effects of automated bed mattress pulsation on the end-expiratory lung impedance measurements. From Reference 22.
End-expiratory lung impedance (EELI) measurements following a bolus of furosemide. EELI continues to increase with no change in distribution of ventilation. From Reference 22.
Summary
Although the use of EIT in the assessment of respiratory function has been studied for more than 3 decades, its clinical use at the bedside is still in its early days. The commercial availability of EIT has dramatically increased the number of studies assessing its abilities to guide clinical practice. There is still significant work that needs to be done to establish the best method of assessing recruitment potential and overdistension and to optimize PEEP both in the acute and in the weaning phase of mechanical ventilation. At this time, most studies have assessed respiratory function in mechanically ventilated subjects; however, more studies should consider the best measurements to use when assessing respiratory function in nonventilated patients. EIT provides valuable insights into how changes to mechanical ventilation or initiating a therapy can affect lung function, becoming widely accepted as a useful bedside tool for this purpose. Clinicians responsible for managing complicated patients should be aware of what EIT has to offer, and researchers should continue to study the best and most practical methods for using this exciting technology at the bedside.
Footnotes
- Correspondence: Thomas Piraino RRT FCSRT FAARC, St. Michael’s Hospital, 36 Queen Street E, Toronto, ON M5B 1W8, Toronto, Canada. E-mail: thomaspiraino{at}gmail.com
Mr Piraino has disclosed relationships with Philips, Aerogen, Dräger, and Fisher & Paykel.
- Copyright © 2022 by Daedalus Enterprises