Abstract
COPD remains one of the leading causes of death worldwide. The impact of smoking and air pollution remain important causative factors. Traditional classification of COPD includes the overlap of emphysema, chronic bronchitis and asthma. More recently the COPDGene definition includes the terms possible, probable and definite COPD. These are supported by findings from exposures, symptoms, spirometry and radiologic imaging. This new approach can lead to earlier diagnosis and modification of risk factors, earlier therapeutic intervention and improved treatments of established disease.
Introduction
Delivering the Thomas L. Petty Memorial Lecture at the 2021 American Association for Respiratory Care International Respiratory Convention was an honor and one of the highlights of my career. Dr Petty was a visionary who helped to shape the field of pulmonary medicine in many ways, including our approach to the definition, diagnosis, and management of COPD.
Dr Thomas Petty and COPD
Dr Petty’s career and publications arced through many components of the COPD world, including pathology,1 definition,2,3 the role of spirometry,4-6 risk factors,7 phenotypes,8,9 polymorbidity,10 long-term oxygen therapy,11 pharmacotherapy,12 epidemiology,13 screening for disease,14,15 and natural history.16 Along the way, he trained or mentored many of today’s leaders in the world of COPD both in the United States and internationally. In 2004, he was also one of the cofounders of the COPD Foundation, which is committed to improving the lives of individuals with COPD.
The History of COPD Classification
The current view of COPD can be traced back to a symposium on chronic lower respiratory diseases that was hosted by Dr Charles Fletcher in 1958.17 The resulting publication from this meeting considered the overlap between chronic bronchitis, asthma, and irreversible obstructive lung disease (defined as persistent narrowing of the airways present for more than one year and unaffected by bronchodilator drugs or corticosteroids).17 Our current unders-tanding of COPD and the overlap between its various components can be traced back to this document even though this predates the term COPD.
By 1966, Dr Fletcher collaborated with Dr Ben Burrows from the United States to examine pathological data from their cohorts of patients with chronic bronchitis and emphysema.18 This work led to the realization that these components often exist in the same patient and represent a shared pathway of disease development and progression. Dr Fletcher also led the effort to better understand the natural history of chronic bronchitis and emphysema by following a group of British men with spirometry every 6 months for 8 years.19 The results of this were summarized in a 1977 report that included the figure of lung function decline, demonstrating an accelerated decline in susceptible smokers that would revert to a more normal decline in former smokers.
In 1987, the American Thoracic Society (ATS) published their first Standards for the Diagnosis and Care of Patients with COPD and Asthma.20 This represents the first official guidance document that used the term COPD and defined it as “a disorder characterized by abnormal tests of expiratory flow that do not change markedly over periods of several months observation. This qualification is intended to distinguish COPD from asthma.”20
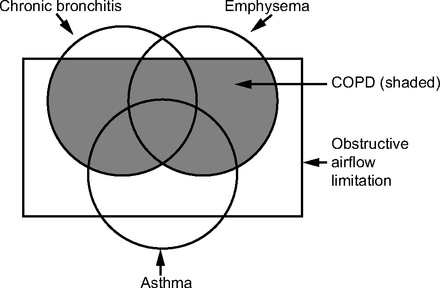
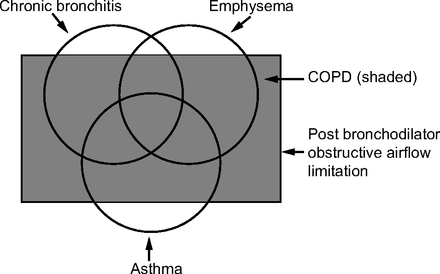
By 1995, an updated version of this document was published21 and now produced the classic figure demonstrating the relation between chronic bronchitis, emphysema, asthma, air flow limitation, and COPD (Fig. 1). These categories and overlaps had their origins in the 1958 symposium hosted by Dr Fletcher.17 The Global Initiative for Chronic Obstructive Pulmonary Disease (GOLD), a collection of global experts in COPD, released their first guideline, global strategy for the diagnosis, management, and prevention of COPD in 2001.22 An important modification in this strategy is reflected in Figure 2, where the pre-sence of post-bronchodilator obstruction (defined as a FEV1/FVC < 70%) now defines COPD. Whereas the GOLD approach has used the fixed ratio of FEV1/FVC < 70% to define obstruction, other authorities, including the ATS and European Respiratory Society (ERS), have advocated the use of the lower limit of normal to define obstruction.23
Modified from the American Thoracic Society standards for the diagnosis and care of patients with COPD.21
Based on the global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease.22
The 2001 GOLD strategy also included a means of classifying severity of COPD based on the degree of lung function impairment (FEV1 as a percentage of predicted) and the absence or presence of respiratory failure. Suggested therapies, which included risk factor reduction, vaccination, short-acting bronchodilators, long-acting bronchodilators, inhaled steroids, pulmonary rehabilitation, oxygen the-rapy, and surgical options, were linked to the degree of severity.22
In 2004, the ATS and ERS published updated standards that were like the GOLD approach regarding diagnosis but took a different approach to classifying severity and treatment options by using symptoms and exacerbation events as key drivers of therapy.24
In 2011, the GOLD committee added structure to this approach with the introduction of GOLD patient categories.25 With this approach, degree of lung function impairment and exacerbation history defined risk with either the modified Medical Research Council (mMRC) dyspnea scale or the COPD Assessment Test score-defined symptoms. This defined 4 groups: A (low risk, fewer symptoms), B (low risk, more symptoms), C (high risk, fewer symptoms), and D (high risk, more symptoms). Treatment was now based on the category in which the patient was classified.
GOLD modified this approach in 2017 such that lung function impairment was still needed to classify a person as having COPD but was no longer part of the process to classify a patient into one of the 4 categories.26 In addition, the importance of reevaluating patients after several months and adjusting therapy was stressed in this and subsequent revisions of the GOLD strategy.
A criticism of these traditional approaches to the diagnosis and classification of COPD is that they find people too late in the disease process, when irreversible damage has already been done.27 This has led to efforts to rethink how COPD is defined and classified with a new approach suggested by the COPDGene consortium28 along with the emerging concept of pre-COPD,29 both of which will be discussed below.
The COPDGene 2019 Classification of COPD
The COPDGene consortium released their new classification system for COPD in 2019.28 This document introduced a fundamental change in how COPD was defined by
Expanding the spirometric criteria for COPD to include both obstructive criteria and restrictive criteria (for which they use the term “Preserved Ratio Impaired Spirometry” or PRISM).
Recognizing the importance of symptoms in the definition and diagnosis of COPD.
Acknowledging that imaging data that look at both airway thickening and emphysema can be a manifestation of COPD.
Adding categories of possible, probable, and definite COPD to the disease classification scheme.
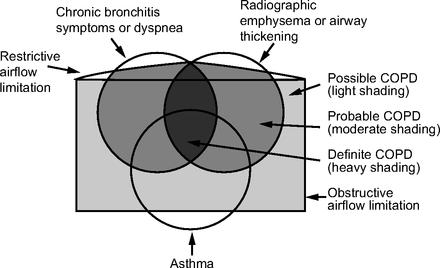
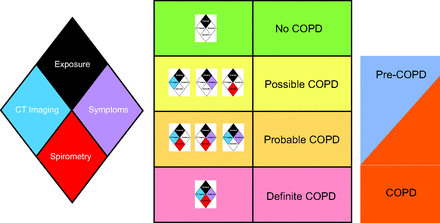
The relationship between the COPDGene classification and the ones discussed previously is depicted in Figure 3, and the COPDGene diamond and classification scheme are depicted in Figure 4.28,30 The components of the diamond, as depicted in Figure 4, are as follows:
Based on the COPDGene 2019 definition of COPD.28 Possible, probable, or definite COPD is represented by the progressively darkening shaded sections.
The relationship between exposures, symptoms, imaging findings, and spirometry in the definition of COPD as defined by COPDGene 2019.28 Patients in the possible or probable COPD categories may be categorized as either COPD or pre-COPD. With permission from Reference 30. CT = computed tomography.
Exposure: The minimum exposure was a 10–pack-year smoking history. Symptoms: Self-report of dyspnea ≥ 2 on the modified mMRC dyspnea score and/or chronic bronchitis (self-reported chronic cough and phlegm).
Computed tomography (CT) structural abnormality: Defined as ≥ 5% emphysema (low attenuation area [LAA] ≤ −950 Hounsfield units [HU]) on inspiratory CT and/or ≥ 15% gas trapping (LAA ≤ −856 HU) on expiratory CT and/or Pi10 (square root of airway wall area for a standardized airway of 10 mm internal perimeter) ≥ 2.5 mm. Spirometry: Patients with abnormal spirometric values (FEV1 < 80% predicted and/or FEV1/FVC < 0.70) were classified as having spirometric evidence of disease.28
This updated classification system has the potential to modify how we approach COPD in both research and clinical scenarios, as described below.
Applications in Research Settings
In the analysis that defined the COPDGene classification approach, Lowe et al28 also examined how this determined important COPD outcomes, such as the loss of lung function and mortality. This involved using the components of the COPD diamond (Fig. 4) to classify patients as possible (2 components), probable (3 components), or definite (4 components) COPD. When going from possible to probable to definite COPD, the odds ratio for accelerated loss of lung function (> 350 mL) increased from 1.26 (95% CI 1.03–1.53) to 1.88 (95% CI 1.52–2.32) to 2.88 (95% CI 2.23–3.71). Similarly, the risk for mortality in these groups increased from 1.28 (95% CI 0.99–1.66) to 1.89 (95% CI 1.48–2.41) to 5.21 (95% CI 4.17–6.52).
The COPDGene approach moves people from “no COPD” in the traditional GOLD approach to one of the COPD categories (possible, probable, definite). When this was evaluated in a recent study, among 5,018 people classified as no COPD under GOLD (of 9,102 in the COPDGene cohort), 3,612 (72%) were reclassified as possible, probable, or definite COPD.31 Decline in lung function and 6-min walk distance, along with an increase in symptoms, was similar between people classified by either the COPDGene or the GOLD classification scheme.31
Applications in Clinical Settings
The COPDGene approach to classification of COPD would be expected to find more patients with COPD than the current GOLD approach. This was demonstrated in the COPDGene study, as noted above,28 and has been confirmed in a real-world study of 1,224 subjects from a large pulmonary practice with a clinical diagnosis of COPD.32 In that study, only 43% would have met COPD criteria by GOLD criteria, and fewer (26%) would have met criteria with the less inclusive Global Lung Initiative criteria.33 They found that 36% had restricted spirometry, and 21% had normal spirometry. In contrast to COPDGene, this analysis also included never smokers with a clinical diagnosis of COPD, of whom only 41% had obstructed spirometry according to GOLD criteria. In this group of never smokers, 43% had restricted spirometry and 21% had evidence of emphysema on CT scan.32 This analysis suggests that clinicians may be ahead of the current international guidelines like GOLD, with a willingness to make a COPD diagnosis in the absence of patients meeting strict spirometric criteria.
The Future of COPD
COPD continues to be a leading cause of death and disease globally.30 Changing this pattern would involve multiple interventions, including decreasing risk factors, finding disease at an earlier time in the disease course, and providing better therapies to those with existing disease.29,34
Finding Early Disease
A major challenge in COPD is that patients are diagnosed long after their disease is established and a great deal of damage has been done.27 The physiological abnormalities that current tools, such as spirometry, detect may occur years after the underlying exposures and biochemical and cellular events that advance the disease process.27 This has been described as analogous to screening for colon cancer by looking for evidence of metastatic disease in the liver. Thus, one of the strategies in COPD is the identification of markers that could be used to find people at an earlier stage in disease development and then apply appropriate interventions to modify disease progression.35 Another concept now being discussed is that of pre-COPD, which has some similarities to the COPDGene expanded definition of COPD in that it includes with symptoms, structural abnormalities, and functional abnormalities as a group of individuals who may go on to develop typical COPD.29
Using Treatable Traits Approach to Improve
Patient Outcomes
In established COPD, outcomes in patients can be improved by focusing on the treatable traits of individual patients.36 These can include pulmonary traits, extrapulmonary traits, and behaviors or other lifestyle factors that can be addressed.36 The trait-specific treatments range from bronchodilators and corticosteroids to cognitive and behavioral therapy, smoking cessation support, and rehabilitation, among many others. The future of COPD therapy may involve better means of measuring these traits using new technologies, such as wearable devices that could monitor patients and alert their care team if they are starting to experience an exacerbation.37
Summary
COPD remains an important global cause of death and disability. Dr Petty’s career highlighted the importance of COPD, including its early detection and treatment. The last 60 years have seen a considerable improvement in our understanding of COPD, including a recent expansion of the group of patients who could benefit from interventions. Dr Petty’s vision helped to get us to where we currently are in our understanding of COPD and will prepare us for the future of COPD.
Footnotes
- Correspondence: David M Mannino MD, Department of Medicine, University of Kentucky College of Medicine, 800 Rose Street, Lexington, KY 40536. E-mail: dmannino{at}uky.edu
Dr Mannino discloses relationships with GlaxoSmithKline and the COPD Foundation.
Dr Mannino presented a version of this article as the Thomas L Petty Memorial Lecture at AARC Congress 2021 LIVE!, held virtually December 3, 2021.
- Copyright © 2022 by Daedalus Enterprises