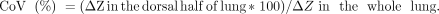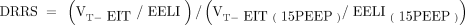Abstract
BACKGROUND: To analyze the role of PEEP on dynamic relative regional strain (DRRS) in a model of ARDS, respective maps were generated by electrical impedance tomography (EIT).
METHODS: Eight ARDS pigs submitted to PEEP steps of 0, 5, 10, and 15 cm H2O at fixed ventilation were evaluated by EIT images. DRRS was calculated as (VT-EIT/EELI)/(VT-EIT[15PEEP]/EELI[15PEEP]), where the tidal volume (VT)-EIT and end-expiratory lung impedance (EELI) are the tidal and end-expiratory change in lung impedance, respectively. The measurement at 15 PEEP was taken as reference (end-expiratory transpulmonary pressure > 0 cm H2O). The relationship between EIT variables (center of ventilation, EELI, and DRRS) and airway pressures was assessed with mixed-effects models using EIT measurements as dependent variables and PEEP as fixed-effect variable.
RESULTS: At constant ventilation, respiratory compliance increased progressively with PEEP (lowest value at zero PEEP 10 ± 3 mL/cm H2O and highest value at 15 PEEP 16 ± 6 mL/cm H2O; P < .001), whereas driving pressure decreased with PEEP (highest value at zero PEEP 34 ± 6 cm H2O and lowest value at 15 PEEP 21 ± 4 cm H2O; P < .001). The mixed-effect regression models showed that the center of ventilation moved to dorsal lung areas with a slope of 1.81 (1.44–2.18) % points by each cm H2O of PEEP; P < .001. EELI increased with a slope of 0.05 (0.02–0.07) (arbitrary units) for each cm H2O of PEEP; P < .001. DRRS maps showed that local strain in ventral lung areas decreased with a slope of −0.02 (−0.24 to 0.15) with each cm H2O increase of PEEP; P < .001.
CONCLUSIONS: EIT-derived DRRS maps showed high strain in ventral lung zones at low levels of PEEP. The findings suggest overdistention of the baby lung.
Introduction
Lung collapse in gravity-dependent lung areas is a hallmark of ARDS that promotes ventilator-induced lung injury (VILI).1,2 Overdistention of the baby lung as a consequence of dependent lung collapse is a well-demonstrated phenomenon related to positive-pressure mechanical ventilation. These nonventilated lung zones act as stress risers on the rest of the lung parenchyma that lead to a local inflammatory response of the baby lung.3 Such pathophysiologic mechanism has been confirmed by complex imaging techniques like intravital alveolar microscopy, synchrotron-based x-ray tomography, computed tomography (CT) scan, and positron emission tomography (PET).4-8
The excessive lung strain, that is, the tidal deformation of lung parenchyma normalized by the reference functional residual capacity (FRC) (strain = VT/FRC), is nowadays considered as one of the main mechanism of VILI.9 This variable is useful for monitoring mechanical ventilation although it has 2 main limitations. First, it is a global variable that does not reflect the typical heterogeneity of lung volume distribution observed in ARDS. Second, the lack of continuous monitoring of FRC/end-expiratory lung volume (EELV) makes lung strain an intermittent measurement, thereby limiting its potential to give real-time information in mechanically ventilated patients with ARDS. Thus, an ideal monitoring tool capable of determining regional lung strain would be valuable at the bedside. Electrical impedance tomography (EIT) is a noninvasive monitoring that includes most of the features of such ideal tool in a breath-by-breath basis.10-12 EIT has the potential to estimate lung strain because a change in impedance is closely related to changes in lung volumes.13,14 Therefore, global lung strain by EIT can be computed as:
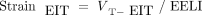
Where VT-EIT and end-expiratory lung impedance (EELI) are the tidal and end-expiratory change in lung impedance, respectively. EELI constitutes a surrogate of ΔEELV when is measured at certain level of PEEP. EELI is measured in arbitrary units. Thus, when some PEEP value is used as reference, it is possible to calculated strain in a pixel-wise fashion that derives to a dynamic relative regional strain (DRRS) map.14 To our knowledge the calculation of DRRS by EIT performed in a model of ARDS has not been described before.
The main objective of this experimental study was to explore the role of EIT to estimate DRRS at different levels of PEEP in a model of ARDS.
QUICK LOOK
Current Knowledge
Gravity-dependent lung collapse in ARDS leads to tidal volume distribution to already ventilated areas. Thus, dependent lung collapse acts to increase stress in the ventral portion of the ventilated baby lung. Such increase in regional lung strain promotes and perpetuates the local inflammatory response.
What This Paper Contributes to Our Knowledge
In an animal model of ARDS ventilated at constant ventilator settings, lung collapse observed at low levels of PEEP increased electrical impedance tomography (EIT) dynamic relative regional strain in the baby lung. These findings were related to the change in physiological variables that describes lung collapse and lung strain. The novel EIT-derived strain maps could help clinicians to guide ventilatory treatments at the bedside.
Methods
This study was performed at the laboratory of the Department of Anesthesiology, Veterinary School, Universidad de Buenos Aires, Argentina. The institutional animal ethical committee board approved the protocol. We have re-analyzed part of published data in which EIT images were successfully obtained in an animal model of ARDS.15 Technical failures in the process of EIT signal downloading were noticed in some PEEP steps of 2 pigs; therefore, complete EIT data sets of only 8 animals were available for image analysis.
Baseline Ventilation, Hemodynamics, and Gas Exchange
The setup and model have been described previously.15 Eight Landrace pigs were anesthetized in supine position and ventilated through a cuffed 7.5 endotracheal tube with a Puritan Bennett 980 (Medtronic, Dublin, Ireland) in volume controlled–continuous mandatory ventilation (VC-CMV) mode using a tidal volume (VT) of 10 mL/kg, a breathing frequency of 24 breaths/min, inspiratory-expiratory (I-E) ratio of 1:2, PEEP of 15 cm H2O, and FIO2 of 1.0. Hemodynamic was assessed by the Vigileo device (Edwards Lifesciences, Irvine, California) from which cardiac index, stroke volume variation, and mean arterial pressure were continuously measured. Arterial blood samples were analyzed with the EPOC system (Siemens, Erlangen, Germany).
Respiratory Mechanics
The FluxMed device (MBMed, Buenos Aires, Argentina) assessed standard and transpulmonary respiratory mechanics. Plateau pressure and total PEEP (PEEPtot = PEEP + PEEPi) were measured during I-E hold maneuvers of 2 s. Driving pressure (ΔP) was calculates as the differences between the above pressures. Static respiratory compliance (CRS) was calculated using standard formula.
An esophageal latex balloon 7-cm long (MBMed) was located at mid-esophagus, and its position was checked with the occlusion method.16 End-expiratory transpulmonary pressure (PL,ee) was calculated as the difference between airway and esophageal pressures with the expiratory hold maneuvers.
Electrical Impedance Tomography
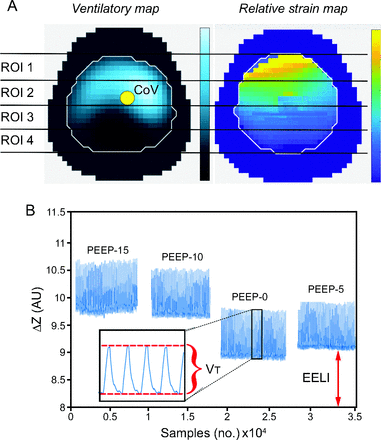
EIT signals were recorded continuously throughout the study protocol using a 32-electrode Swisstom BB2 device (Swisstom, Landquart, Switzerland). The sampling rate used was 47.7 Hz, and EIT lung images contained 32 × 32 pixels. EIT data were continuously recorded on a laptop and analyzed offline using MATLAB R2018b (MathWorks, Natick, Massachusetts). We defined the lung area in EIT images as all pixels showing a tidal impedance change (ΔZ) of > 10% of the maximum amplitude at any PEEP level. Ventilatory maps were segmented using the classical 4 horizontal parallel regions of interest (ROIs) (Fig. 1A): ROI 1 (ventral), ROI 2 (central ventral), ROI 3 (central dorsal), and ROI 4 (dorsal).17 The following EIT-derived parameters were calculated:
EELI is the absolute value of the impedance at the end of expiration; it is a dimensionless variable. Identification of pixel end-expiratory minima before and after a change in ventilator settings (change in PEEP) creates a functional EIT image of the local changes in EELV.
Center of ventilation (CoV) represents the distribution of overall ventilation placed in one single point within the EIT ventilatory map (Figure 1A).18 CoV is expressed as a percentage of the anteroposterior extension of the identified lung region, from 0% (ventilation at the most ventral lung areas) to 100% (ventilation at the most dorsal zones). CoV was calculated as18

DRRS was calculated pixel-wise dividing the change in electrical impedance during VT by EELI for each pig and for each PEEP level. These data were referenced to PEEP at 15 cm H2O, where atelectasis assessed by lung ultrasound was minimal, compliance maximal, and PL,ee > 0 cm H2O in our pigs.15 A strain maps was then built as observed in Figure 1 A and B. For each ROI, DRRS was calculated as average strain of the whole ROI at each individual PEEP level as follow:14
Analysis of electrical impedance tomography (EIT) images. A: Ventilatory and relative strain maps are displayed with their code-color scale at the right hand (lighter color = more ventilation and strain; darker color = less ventilation and strain, respectively). Images were segmented in 4 horizontal regions of interest (ROIs). The center of ventilation (CoV) is represented by a yellow dot. B: Randomized changes in PEEP during the protocol. Changes in tidal impedance (VT-EIT) and end-expiratory lung impedance (EELI) were used to calculate lung strain in a pixel-wise fashion.
EIT images were divided into 4 ROIs (ROI 1 is the most ventral region, and ROI 4 is the most dorsal one) as mentioned above.
Six rows of pixels formed each region.
The calculations were performed on individual ROIs, where the number of pixels in each one of these regions varies from pig to pig.
The strain per pixel in each ROI was calculated as = (Σ VT/EELI/VTref/EELIref)/number of pixels, using PEEP 15 cm H2O as a reference:

Protocol
A 2-hit experimental ARDS model was performed by lung lavages followed by 2 h of injurious ventilation. The ARDS model was confirmed by a PaO2/FIO2 ≤ 200 mm Hg with the presence of bilateral dorsal atelectasis assessed by lung ultrasound images using a linear 8–12 MHz probe (MicroMaxx echograph; Fujifilm Sonosite, Bothell, Washington).19
The protocol started recording baseline ventilation as described above. Randomized PEEP steps of 0, 5, 10, and 15 cm H2O at constant ventilation were applied for 10 min each. After each PEEP step, the ventilator’s circuit was disconnected for 15 s, followed by baseline ventilation for 10 min. Lung ultrasound confirmed the presence of lung collapse before any protocol step to maintain similar baseline conditions.
Electrical impedance and respiratory mechanics were recorded continuously, and the last 2 min of each PEEP step were analyzed. Arterial blood gas analysis was done toward the end of each PEEP step.
Statistical Analysis
Comparison of variables on different levels of PEEP was performed with repeated-measures analysis of variance with Bonferroni post hoc test. Continuous variables are expressed as mean ± SD. The relationship between EIT variables (EELI, CoV, and DRRS) and PEEP was assessed with mixed-effects models using lme4 R package (R Foundation for Statistical Computing, Vienna, Austria).20 EIT measurements and calculations were used as dependent variables. PEEP was included in the model as fixed-effect variables. The animal was introduced in these models as random intercepts. Additionally, in some cases the ROI value was also added to evaluate the interaction with the mechanical parameters as fixed factor.
A P value ≤ .05 was considered statistically significant. Data analysis and graphics were performed with R project software version 3.6.1 (R Foundation for Statistical Computing).
Results
The protocol was completed in 8 pigs (30 ± 9 kg) in which EIT images were successfully recorded and hemodynamic variables remained stable. Table 1 shows main studied variables taking as reference the PEEP level of 15 cm H2O where mean PL,ee > 0 cm H2O. Lung mechanics and gas exchange variables improved with higher levels of PEEP. Comparing zero PEEP versus 15 PEEP, there was an increase in CRS (63%, P < .001) and PaO2 (383%, P < .001) and a decrease in airway DP (56%, P < .001) and PaCO2 (15%, P = .003).
Main Studied Parameters During the Protocol
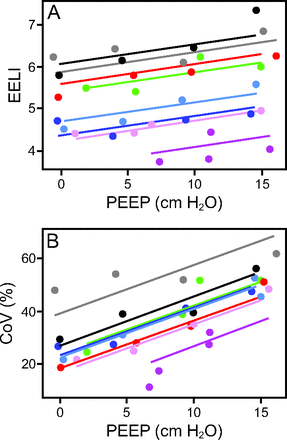
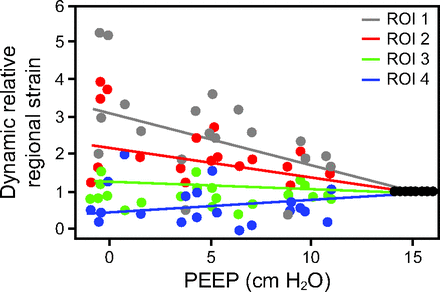
Table 2 depicts the main results of the mixed-effect regression models. EELI significantly increased with larger PEEP values as observed in Figure 2A. Figure 2B illustrates the relationship between PEEP and CoV, showing a shift in the CoV to the dorsal regions with increasing PEEP values. PEEP, ROI, and their interaction show a statistically significant effect on DRRS (Table 2). The interaction term explains the decreasing value of the slopes in PEEP and DRRS regression lines according to ROI as illustrated in Figure 3.
Changes in end-expiratory lung impedance (EELI) and center of ventilation (CoV) during the protocol. Changes in end-expiratory lung impedance (EELI, expressed in arbitrary units [AUs]) (A) and in center of ventilation (CoV) (B) at different PEEP.
Electrical impedance tomography (EIT)–derived regional strain during the protocol. EIT-derived regional strain normalized with data obtained at 15 cm H2O of PEEP.
Summary of Mixed-Effect Linear Regression Models Fixed Effects
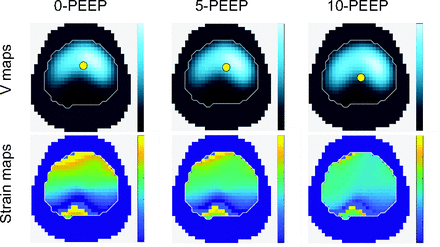
Figure 4 depicts the ventilatory and DRRS maps in one animal as PEEP increases from 0–10 cm H2O.
Ventilatory and dynamic relative regional strain maps in one representative animal. The center of ventilation is depicted by a yellow dot into the ventilatory maps (V maps).
Discussion
The main result of this experimental study was that lung collapse observed at low levels of PEEP decreased EELI, moved CoV ventrally, and increased DRRS in ROIs 1–2 on EIT images. This finding was associated with impaired lung mechanics and marked hypoxemia. The use of higher levels of PEEP partially reverted those negative effects on lung mechanics and gas exchange, making ventilation more homogeneous and decreasing ventral DRRS.
The clinical measurement of FRC has become available in a few commercial ventilators. As PEEP is aimed at restoring FRC and at improving lung function, this new technical bedside feature of FRC measurements enables a goal-directed titration of PEEP and the calculation of global lung strain. The DRRS maps, however, go one step further by providing estimates of lung strain on a regional and breath-by-breath basis, hoping that DRRS will help care providers to personalize protective ventilation for their patients.
The role of lung collapse as stress raiser in the baby lung ventilating at low PEEPs has been previously described.3-8 Many animal studies described in detail such pathophysiological sequence that leads to mechanical lung injury. Tsuchida et al3 showed in injured rat lungs that mechanical ventilation with low PEEP caused more histological injury in ventilated than in nonventilated areas. Similar findings were found by Cereda et al21 in injured rat lungs using hyperpolarized magnetic resonance images. They found that atelectasis in dependent lung increased apparent diffusive coefficient of 3He (surrogate of overdistention) in aerated lung zones. This overdistention was most prominent at zero of PEEP and was reduced when PEEP recruited atelectasis.
In larger lung-injured animals, Retamal et al22 built 3D maps of lung strain with CT scan images taken at end inspiration and end expiration. The authors found prominent inflammation of ventilated areas using PET/CT imaging of 18Fluoro-2-deoxy-D-glucose (18F-FDG) that was spatially correlated with high local lung strain. Regional lung strain was also appreciated at the microscopic level by Rausch et al5 using synchrotron-based x-ray tomographic microscopy in rat lungs.
Similar results have been described in humans with ARDS. In a seminal paper, Terragni et al23 showed greater end-tidal overinflation by CT scan images in the one third of subjects with extensive atelectasis in dependent lung areas compared to the other two thirds of subjects with less lung collapse. Bellani et al6 using PET/CT 18F-FDG showed a heterogeneous spread of inflammation within lungs, although one third of them showed inflammation in normally ventilated lung areas.
Our results are in line with the above body of knowledge. We showed that lung collapse was associated with impaired lung mechanics and marked hypoxemia (Table 1). Negative PL,ee together with low compliance, hypoxemia, and high DP suggests alveolar instability at these low PEEPs levels.15,23,24 EIT-derived DRRS maps together with CoV displacement located higher strain in ventral areas of the lungs at low PEEPs (Figs. 2 and 3).
These findings are similar to the Spadaro et al25 study in ventilated subjects with EIT images, which showed that CoV displaced to ventral areas when decreasing PEEP from 15 (49.4%) to 5 cm H2O (41.9%) due to dependent lung collapse. Furthermore, in our previous experimental study, we demonstrated that lung collapse found at low PEEPs was associated not only with impaired lung mechanics but also with an increase in alveolar dead space, a surrogate of alveolar overdistention. In our animals, the use of higher levels of PEEP partially reversed these negative effects on lung distention, making ventilation more homogeneous.
As EIT is a technique based on relative changes in impedance, the absolute strain cannot be calculated because absolute EELV must be known. Therefore, we have used the EELI as a surrogate of EELV (Fig. 1B). We normalized EIT strain to values observed with a PEEP of 15 cm H2O because under this condition lung collapse was minimized, oxygenation and lung mechanics were optimal, and a PL,ee > 0 cm H2O in most animals.15 PL,ee above zero is related to a lung condition where lung collapse is minimized and the mechanism of stress raisers would be limited.26
Local strain can increase several times above the global value according to the model of Mead et al27 and the results obtained by Rausch et al5 using computer simulations of real alveolar synchrotron-x-ray tomography data. Thus, global strain could not represent the real magnitude of lung units deformation during a mechanical breath, emphasizing the potential interest of regional assessment with tools such as EIT-derived DRRS maps.
Limitations
The 2-hit model, as any other animal model of ARDS, cannot reproduce the complexity of human ARDS.28 In addition, the use of nonprotective ventilation with zero PEEP and/or VT of 10 mL/kg is not consistent with current recommendations for the management of mechanical ventilation in this patient population. The animal model in conjunction with nonprotective ventilatory settings was chosen in the original study to examine deadspace at different degrees of lung overdistention.15 Therefore, it is emphasized that ventilation at zero PEEP and high VT is harmful for human patients, and the extrapolation of our results obtained with this 2-hit model into the clinical context must be done with caution. Our findings represent the results of a proof-of-concept study, and thus interpretation should not be centered on the ARDS model or lack of lung-protective ventilation but on the novel EIT-derived strain maps and their potential to reveal changes in regional lung strain. Further studies are warranted, which should determine the value of DRRS maps in the context of standard lung-protective ventilation within the clinical setting.
Another pitfall of our study was the lack of an accepted standard to compare our EIT-derived DRRS maps. There is a new and elegant CT scan–based analysis of regional lung strain described by Hurtado et al that was not available at the time to design this study.29-31 This analysis gives absolute regional strain values when comparing volumetric CT images between inspiration-expiration. The same group has recently described a close correlation between the global strain measured by CT and ΔZ in patients with ARDS (r2 = 0.855, P < .001).31 Thus, knowing that ΔZ is closely related to changes in tidal lung volumes13 and global strain,31 the possibility of calculating DRRS at the bedside would be a reality.
Conclusions
EIT-based dynamic relative strain maps showed more lung strain in ventral pulmonary areas at low PEEPs. Such high strain was related to other physiological variables that reflect lung collapse and overdistention. Therefore, these findings in an experimental model of ARDS suggest possible overdistention of the baby lung with low PEEP levels.
Footnotes
- Correspondence: Gerardo Tusman MD, Department of Anesthesiology, Hospital Privado de Comunidad, Mar del Plata, Argentina. E-mail: gtusman{at}hotmail.com
See the Related Editorial on Page 1061
Dr Tusman wrote a patent regarding electrical impedance tomography and lung strain. Dr Madorno is partner and manager of MBMed SA. Mr Gogniat is currently employed by Medtronic Argentina. The remaining authors have disclosed no conflicts of interest.
This study was performed at the Hospital Veterinario Universitario de la Universidad de Buenos Aires, Buenos Aires, Argentina.
- Copyright © 2022 by Daedalus Enterprises