Abstract
BACKGROUND: Inspiratory muscle training (IMT) strategies can reduce ICU length of stay and optimize recovery in critically ill patients. Our objective was to compare IMT combined with spontaneous breathing with T-piece in tracheostomized subjects.
METHODS: Tracheostomized critically ill subjects who were ready to wean were selected and randomly allocated to one of 2 groups: electronically-assisted IMT (EIMT) or spontaneous breathing with T-piece. Electronically assisted IMT was delivered using 30% of maximal inspiratory pressure (manual EIMT or automatically adjusted loads). The following variables were analyzed: ICU length of stay, weaning time, maximal inspiratory pressure, rapid shallow breathing index, pressure (cm H2O), power (W), flow (L/s), volume (L), and energy (J).
RESULTS: A total of 132 patients were assessed; 104 subjects were enrolled with EIMT, n = 51 (automatic EIMT, n = 25 and manual EIMT n = 26), or spontaneous breathing with T-piece group, n = 53. The Acute Physiology and Chronic Health Evaluation II score was significantly higher (P = .02) in subjects in the manual EIMT group. Weaning time did not differ significantly between groups (8.55 ± 6.48 d and 10.86 ± 6.48 d, EIMT and spontaneous breathing with T-piece group, respectively; P = .23). Weaning success rates (75%) were lower in the manual EIMT group. Invasive mechanical ventilation time was longer but not significantly different (P = .21) in the spontaneous breathing with T-piece group. Maximal inspiratory pressure was significantly higher in the spontaneous breathing with T-piece and the automatic EIMT groups (P < .001 and P = .007, respectively). Pressure, power, and energy values were significantly higher in the manual EIMT group (P < .001, P = .003, and P = .003, respectively).
CONCLUSIONS: IMT modalities in this trial had no significant impacts on weaning time or successful weaning rates.
Introduction
Weaning from mechanical ventilation is a routine procedure in critically ill patients in the ICU. According to Esteban et al,1 time spent in the weaning process accounts for more than 40% of the total duration of invasive mechanical ventilation. Failed weaning occurs in approximately 12–50% of cases requiring mechanical ventilation2 and may be due to respiratory muscle weakness, diaphragm muscle dysfunction, critical illness polyneuropathy, nutrient-drug interactions, recurrent sepsis, rhabdomyolysis, and/or phrenic nerve injury.2-7
Controlled mechanical ventilation contributes to ventilation-induced diaphragm dysfunction. This condition may occur 6 h after invasive ventilation initiation and tends to become progressively worse in patients ventilated for > 6 d. Shortened muscle action potential and decreased contractility lead to disuse-induced muscle weakness.8 Several well-established, interrelated processes have been illustrated by Dress et al,9 including malnutrition-related muscle wasting, sepsis-related oxidative stress, reduced protein synthesis, and increased proteolysis and mechanical ventilation, which compromises diaphragm activity, leading to muscle atrophy. In a study using muscle biopsy to investigate contractile strength, Van den Berget al,10 found that reduced muscle thickness was the primary structural change and that this change was unrelated to mitochondrial dysfunction or oxidative stress.
Muscle training protocols developed for critically ill patients have been addressed in several review articles. Threshold inspiratory muscle training (IMT) is the most popular intervention used in this population, and the following effects have been described: increased exercise tolerance, increased muscle strength and endurance, reduced weaning time from mechanical ventilation, and reduced hospital length of stay. Threshold IMT is a mechanical resistor that uses a spring-loaded valve to generate air flow resistance, measured in cm H2O. Training quality or results per session cannot be determined.2-4,11
The first randomized clinical trial investigating IMT using the Powerbreathe electronic device in critically ill ICU subjects was published by Tonella et al.7 In that trial, hemodynamic and respiratory variables were used to assess device applicability and safety. Powerbreathe IMT was thought to be a safe alternative for tracheostomized critically ill patients who required prolonged mechanical ventilation (PMV) and were difficult to wean.7
According to Bisset,12 early proactive respiratory muscle rehabilitation is both feasible and effective in ICU settings. Targeted isokinetic respiratory muscle training is recommended for optimal recovery. Candidates for IMT must be alert and cooperative and may be divided into 2 groups: mechanical ventilation dependent and recently weaned from mechanical ventilation.
ICU patients often require IMT. In these patients, muscle training variables can be successfully controlled using isokinetic load devices. This study set out to compare the outcomes of spontaneous breathing with T-piece alone or combined with 2 IMT modalities using electronic devices in tracheostomized critically ill subjects.
QUICK LOOK
Current Knowledge
Inspiratory muscle training (IMT) can be used in critically ill patients with respiratory failure induced by prolonged mechanical ventilation to reduce ICU stay and optimize recovery. Consensus regarding optimal IMT timing is lacking, and impacts of different training loads on weaning time have not been accurately determined.
What This Paper Contributes to Our knowledge
In this study, ICU length of stay and weaning time did not differ between tracheostomized subjects submitted to automatic or manual IMT with electronic device or spontaneous breathing with T-piece. Maximum inspiratory pressure (PImax) change was not associated with shorter mechanical ventilation or weaning time. Further studies are warranted to determine the appropriate load, number of series, and training goals.
Methods
This was a prospective, randomized, interventional comparative study conducted at the University Hospital (HC Unicamp) of Campinas State University. The following adult ICUs were involved: internal medicine, trauma, surgery, and neurology. This project was approved by Unicamp’s Institutional Review Board (Opinion No. 16519913.4.5404) and registered in the Brazilian Clinical Trial Registry (ReBEC; registration No. U1111-11563177).
The sample comprised male and female tracheostomized subjects age 18 y or older admitted to HC Unicamp ICUs. All subjects were receiving invasive mechanical ventilation (Hamilton RAPHAEL, Hamilton Medical, Bonaduz, Switzerland; Dräger Evita, Dräger, Lübeck, Germany; Newport e360, Medtronic, Dublin, Ireland) and met criteria of readiness for weaning. Inclusion criteria are listed in Table 1.
Inclusion Criteria
Selected subjects were randomly allocated to one of 2 groups: IMT with an electronic Powerbreathe device (EIMT) (IMT Technologies, Birmingham, United Kingdom) or spontaneous breathing with T-piece. Randomization was achieved using allocation concealment (sequentially numbered, opaque sealed envelopes). Subjects allocated to the EIMT group were further categorized into subgroups according to the Glasgow coma scale (Glasgow coma scale) score, as follows: Glasgow coma scale score ≥ 9, automatic EIMT group; Glasgow coma scale score ≤ 8, manual EIMT group.
Subjects were submitted to respiratory physiotherapy (institutional standard of care) consisting of bronchial hygiene and tracheal and oral cavity suction maneuvers in the semi-seated position (head of bed raised to 30°) and physical training consisting of bedside sitting and active and active-assisted exercises. Devices used for respiratory muscle training and assessment were connected using a disposable hygroscopic filter with FIO2 set to 1.0 while subjects were reconnected to the mechanical ventilator. Ventilatory and hemodynamic variables were monitored during clinical assessment and physical therapy sessions using a multiparameter monitor (IntelliVue, Philips, Amsterdam, the Netherlands). The following variables were monitored: breathing frequency (breaths/min), SpO2, mean blood pressure (MBP), and heart rate.
Subjects with sufficient level of consciousness to understand and respond to verbal commands (Glasgow coma scale score > 8) were allocated to automated EIMT. In this mode of ventilation, load is automatically adjusted according to the maximal effort exerted by patients during the first 2 breaths of each training session. Subjects who were not able to cooperate or understand instructions given prior to and during training sessions (Glasgow coma scale score ≤ 8) were allocated to manual EIMT. Subjects in this group were submitted to a load corresponding to 30% of PImax, with daily increments of 10%.
In the EIMT group, a KH2 electronic device (IMT Technologies) connected to a notebook (Samsung Ultrabook, Samsung, Seoul, South Korea) equipped with the Breathe-Link software (IMT Technologies) was used. Training sessions were held twice daily (morning and afternoon), 7 d per week. Sessions consisted of 30 repetitions guided by the respiratory physiotherapist. Repetitions were divided into 3 series of 10 at 1-min intervals. In both groups, EIMT was maintained until subjects could breathe on their own for 48 h. EIMT was temporarily withheld in the following cases: 20% increase in MBP and heart rate relative to baseline or sustained hemodynamic instability with MBP ≤ 80 mm Hg or ≥ 110 mm Hg and/or heart rate ≤ 60 or ≥ 120 beats/min. In subjects requiring vasoactive agent doses > 5 μg/kg/min, EIMT was temporarily discontinued and reinstituted after hemodynamic stabilization. Whenever family or multidisciplinary team members decided to withdraw treatment (limitation of therapeutic effort), EIMT was discontinued and the patient excluded.
Spontaneous breathing with T-piece (standard of care) was combined with the EIMT protocol. Subjects in this sample were submitted to spontaneous breathing with T-piece (either alone or in combination with EIMT). Subjects who were able to tolerate pressure support of 10 cm H2O on spontaneous mode with FIO2 ≤ 0.6, PEEP ≤ 10 cm H2O, breathing frequency ≤ 30 breaths/min, and SpO2 ≥ 90% received oxygen delivered via a T-piece connected to a tracheostomy tube by the physiotherapist in order to achieve a target SpO2 ≥ 90%. T-piece ventilation time was progressively increased according to the following parameters: no signs of respiratory discomfort (use of accessory muscles, nose flaring, paradoxical respiratory pattern, and sweating), breathing frequency ≤ 30 breaths/min, SpO2 ≥ 90%, MBP ≥ 80 mm Hg or ≤ 110 mm Hg, and heart rate ≥ 60 beats/min or ≤ 120 beats/min. Multidisciplinary team members were instructed to monitor signs of respiratory discomfort and inform physiotherapists of the need to resume mechanical ventilation. After reinstitution of mechanical ventilation, settings for pressure support ventilation were adjusted to reduce discomfort. In unresponsive cases, assisted ventilation (controlled mode) was delivered for a minimum period of 6 h in order to allow respiratory muscle rest and the need of an algosedation was determined. The same outcome variable (weaning time, ie, time from tracheostomy to achievement of continuous spontaneous breathing for 48 h) was evaluated in the spontaneous breathing with T-piece group.
Subjects in this study underwent specific IMT and were followed until weaning. Successful weaning was defined as the ability to breathe continuously for 48 h without support from a mechanical ventilator. Weaning failure was defined as the need to resume mechanical ventilation within 48 h of withdrawal or transfer to general wards on mechanical ventilation. Weaning duration was calculated as the time/d required to achieve continuous spontaneous breathing for 48 h after having been weaned off sedation for 24 h. Stay in ICU was also assessed in both groups.
PImax and the rapid shallow breathing index (RSBI) were assessed once daily and compared to baseline. These variables were measured using a digital manovacuometer (MVD 300, GlobalMed, Porto Alegre, Rio Grande do Sul, Brazil) connected to a unidirectional valve with 20 s occlusion time and a ventilometer (Wright Mark 8, KoKo, Longmont, Colorado), respectively. Measurements were repeated 3 times at 1-min intervals and the largest value selected for analysis. Data were collected from enrollment to completion of 48 h of continuous spontaneous breathing with T-piece.
Plots and numerical data representing subject performance variables were generated for every EIMT session. The following variables were analyzed: pressure (cm H2O), power (W), flow (L/s), volume (L), and energy (J). Baseline and final values were compared. Training load was compared between EIMT subgroups.
Statistical analysis was performed using R software (R Core Team, 2016; R: A language and environment for statistical computing, R Foundation for Statistical Computing, Vienna, Austria). Data were non-normally distributed. Therefore, nonparametrical tests were used. The chi-square, the Pearson chi-square, and the Fisher exact tests were used to examine differences between 2 independent groups. The Kruskal-Wallis rank-sum test was used to examine differences between 3 independent groups. The Wilcoxon test was used to examine before and after differences. The Mann-Whitney test was used to examine differences between 2 independent groups. The level of significance was set at 5%.
Results
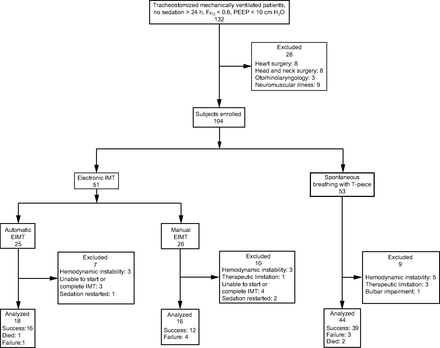
From October 2013–November 2016, 132 patients were assessed. Of these, 28 failed to meet inclusion criteria and were excluded. The final sample comprised 104 subjects who were randomly allocated to one of 2 groups, as follows: EIMT (n = 51) and spontaneous breathing with T-piece (n = 53). Subjects in the EIMT group were further divided into subgroups (automatic EIMT and manual EIMT subgroups, 25 and 26 subjects, respectively). Seven out of 25 subjects in the automatic EIMT group were excluded due to hemodynamic instability, inability to tolerate IMT, or resedation (3, 3, and one subject, respectively). Ten out of 26 subjects in the manual EIMT group were excluded due to hemodynamic instability, limitation of therapeutic effort, inability to tolerate IMT, or resedation (3, one, 4, and 2 subjects, respectively) Figure 1.
Flow chart. IMT = inspiratory muscle training.
Subject characteristics (sex, anticipated mortality, diagnosis, type of ICU, comorbidities, and reason for orotracheal intubation) are shown in Table 2. The Acute Physiology and Chronic Health Evaluation II (APACHE II) score differed significantly (P = .02) between groups. Subjects in the manual EIMT group achieved the highest APACHE score (18.67 ± 8.04), with anticipated mortality of 33.6 ± 22.0%. Most subjects (41.67% −11 subjects) in the manual EIMT group were in the internal medicine ICU. However, type of ICU did not differ significantly (P = .79) between groups. Overall, 39.39% of subjects (41) had been diagnosed with a neurologic condition. Neurologic diagnosis prevailed in all groups, with no significant differences (P = .64) between groups.
Etiology and Population
Analysis of weaning time and weaning success/failure rates in subjects in this sample (spontaneous breathing with T-piece = 44; automatic EIMT group = 18; manual EIMT group = 16) revealed longer weaning time (10.86 ± 8.77 d) in subjects in the spontaneous breathing with T-piece group and lower successful weaning rates (75%) in the manual EIMT group. However, these differences were nonsignificant (P = .45) (Table 3).
Comparison of Weaning Time and Success Rate Between Groups
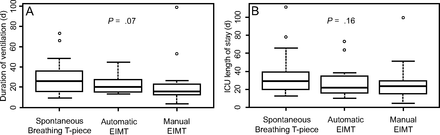
Mechanical ventilation time and ICU stay did not differ significantly (P = .07 and P = .16, respectively) between subjects in this sample. Mechanical ventilation time was longer in the spontaneous breathing with T-piece group, followed by the automatic EIMT and the manual EIMT group: 24.5 (15.75–35.25) d, 19.0 (15.25–26.50) d, and 14.5 (12.00–21.75) d, respectively, Figure 2.
Groups: spontaneous breathing with T-piece, automatic EIMT, manual EIMT. Kruskal-Wallis test, P ≤ .05. Median levels (center line of box), 25–75% CIs (upper and lower borders of the box); upper line (maximum value); bottom line (minimum value); dots (outlier points).
In this study, PImax increased significantly in the spontaneous breathing with T-piece and the automatic EIMT groups (P < .001, P = .007, respectively), whereas RSBI decreased significantly in the spontaneous breathing with T-piece group (P = .03) (Table 4).
Comparison of Mean Starting and Final Measurements for Maximal Inspiratory Pressure and Rapid Shallow Breathing Index Between the Groups
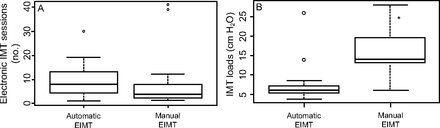
The number of EIMT sessions did not differ significantly between groups, 7.5 (4.0–12.5) sessions and 3.5 (2.0–8.0) sessions, automatic and manual EIMT group, respectively; P = .09. Median EIMT load was significantly higher in the manual relative to the automatic EIMT group, 14.0 (13.0–19.5) cm H2O and 5.74 (5.3–7.1) cm H2O, respectively; P < .001, Figure 3.
EIMT = electronic inspiratory muscle training; IMT = inspiratory muscle training. *Wilcoxon test = *P ≤ .05. Median levels (middle line of box), 25–75% CIs (upper and lower borders of the box); upper line (maximum value); bottom line (minimum value); dots (outlier points).
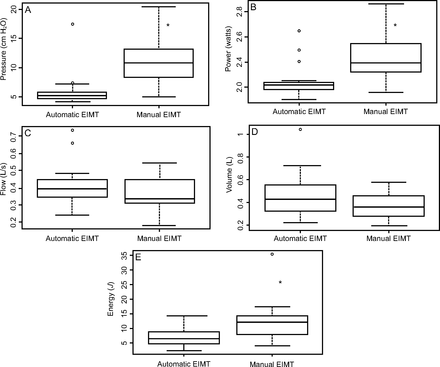
Median and IQR of pressures generated during IMT were significantly higher in the manual relative to the automatic EIMT group, 10.83 (8.45–13.03) cm H2O and 5.16 (4.69–5.60) cm H2O, respectively; P < .001. Likewise, median power values differed significantly between both groups, 0.38 (0.32–0.53) W and 0.21 (0.18–0.23) W, manual and automatic EIMT group, respectively; P < .001. Inspiratory flow during IMT did not differ significantly between groups, 0.39 (0.35–0.44) L/s and 0.33 (0.30–0.44) L/s, manual and automatic EIMT group, respectively; P = .03.
Tidal volume during IMT sessions was higher in the automatic relative to the manual EIMT group, 0.42 (0.32–0.55) L and 0.35 (0.28–0.45) L, respectively. However, these differences were nonsignificant (P = .12). Median and IQR of energy expenditure during EIMT were significantly higher in the manual relative to the automatic EIMT group, 12.24 (8.51–13.92) J and 6.53 (5.05–8.58) J, respectively; P = .003, Figure 4.
Groups: automatic EIMT and manual EIMT. *Wilcoxon test = *P ≤ .05. Median levels (middle line of box), 25–75% CIs (upper and lower borders of the box); upper line (maximum value); bottom line (minimum value); dots (outlier points).
Discussion
Subjects in this sample were homogeneous regarding overall and demographic characteristics except APACHE II score (Table 2). In a study conducted by Jarmuła et al13 with 130 subjects submitted to PMV, APACHE II score was not correlated with weaning outcomes. According to authors of that study, clinical outcomes in these subjects were affected by several respiratory and non–respiratory factors rather than a single factor. In contrast, subjects allocated to manual EIMT in this sample had higher APACHE II scores and lower successful weaning rates relative to remaining groups (75% vs 88%, respectively).
In studies assessing IMT and weaning time in tracheostomized individuals, the time required to wean subjects from mechanical ventilation ranged from 10–11 d or 16–17 d (Bissett et al6 and Pascotini et al,5 respectively). These findings emphasize differences in duration of mechanical ventilation between clinical and neurologic cases. As reported by Pascotini,5 38% of subjects in this sample was diagnosed with neurological conditions. Different from our team, Faez et al14 recommend early tracheostomy (ie, < 48 h after intubation) in patients with severe head trauma.
In this sample, 45% of subjects in the spontaneous breathing with T-piece group and 39% of subjects overall suffered from neurological dysfunctions. Trapp et al15 investigated diaphragm electric activity during standard nebulization in neurologic subjects and concluded that diaphragmatic electric activity may precede clinical manifestations of distress. The fact that criteria adopted in this study were based on clinical signs rather than diaphragm electromyography may explain longer weaning times reported.16
In this study, additional IMT protocols (manual or automatic) did not decrease weaning time relative to the institutional standard of care. Of notice, similar objective criteria for weaning interruption and resumption of mechanical ventilation were applied to all groups (signs of respiratory discomfort perceived by the medical team, such as use of accessory muscles, nose flaring, paradoxical respiratory pattern, and sweating combined with breathing frequency ≤ 30 breaths/min, SpO2 ≥ 90%, MBP 80 ≥ mm Hg or ≤ 110 mm Hg, and heart rate ≥ 60 beats/min or ≤ 120 beats/min).
In weaning time analysis, calculation of the power for the Mann-Whitney test with groups means and SD was d = 0.28, with a test power of 0.21. In order to achieve a power of 0.8 for this variable, a sample with 444 subjects would be required. This sample size was not achieved in this study.
Stay in ICU did not differ between groups (P = .16). Likewise, mechanical ventilation time was equally long in all groups (P = .07). Aside from the diaphragm muscle, other factors may contribute to PMV and difficult weaning, such as critical illness polyneuropathy, nutrition-drug interactions, and recurrent sepsis.2-7 Three different IMT strategies were used in this study, with no significant impacts on ICU length of stay.
The decision to implement IMT was not based on baseline PImax. Mean baseline PImax of −40 cm H2O in all groups suggests absence of muscle weakness (Medrinal et al).17 In this sample, PImax values were lower than values obtained using the prediction equation for normal PImax for sex and age (Evans and Whitelaw).18 Hence, according to that equation, all subjects would be mechanical ventilation dependent. Significant PImax increase in the spontaneous breathing with T-piece and the automatic EIMT group was not associated with shorter mechanical ventilation and weaning times (ie, in this trial, PImax had no significant impacts on ventilation outcomes).
Weaning success rates in this sample ranged from 75–88%. High weaning success rates may have reflected normal baseline RSBI (81.65 ± 38.92 breaths/min/L). According to Yang and Tobin,19 baseline values < 105 breaths/min/L may anticipate weaning success. Subjects in the spontaneous breathing with T-piece group had a significant (P = .03) decrease in RSBI. However, RSBI had no significant impacts on weaning time. Bien et al20 investigated the value of RSBI as a predictor of weaning in critically ill ICU subjects and concluded this variable is more useful as a parameter to estimate muscle endurance than to predict weaning success.
Subjects undergoing IMT with an electronic device were submitted to an equivalent number of sessions with different loads according to specific training principles. In the manual EIMT group, load calculation was based on methods reported by Cader et al21 and Tonella et al7 and adjusted to 30% of PImax, with daily increment of 10% increase in critically ill ICU subjects. Similar IMT frequency (30 cycles) was used by Kulkarni et al, 2010,22 in a study with subjects undergoing Powerbreathe IMT.
Loads used in the manual EIMT group were significantly higher (P < .001). In this group, loads were calculated according to PImax measured using a unidirectional valve, whereas loads applied in the automatic EIMT group were estimated according to subject cooperation and maximal effort. Different from other studies, in which load was adjusted according to patient effort (Martin et al23 and Bissett et al6), automated Powerbreathe IMT was not PImax dependent, and loads did not vary in this study. In the automatic EIMT group, IMT with loads consistent with daily respiratory muscle effort resulted in higher PImax and lower RSBI. However, these effects were not significantly different relative to spontaneous breathing with T-piece or manual Powerbreathe IMT.
In this study, energy expenditure and power during IMT (ie, muscle strength and speed of muscle contraction) were significantly higher in the manual relative to the automatic EIMT group. The need to spend more energy to overcome higher loads translates into lower speed of muscle contraction and higher power.24 High pressures imposed by manual Powerbreathe IMT may have contributed to slower muscle contraction, increasing the power required to overcome resistance and hence energy expenditure.24
This study compared 3 different IMT techniques in tracheostomized critically ill ICU subjects. In the manual EIMT group, load adjustment according to PImax resulted in higher training loads and energy expenditure but had no positive impacts on weaning time. This IMT modality may be an alternative in non–collaborative subjects. However, further studies are warranted for accurate determination of training load and frequency as well as training goals (ie, muscle strength, muscle endurance, or both). In the automatic EIMT group, IMT load was determined according to individual maximal daily effort, regardless of baseline PImax. Use of different training loads and measurement methods between groups in this study may have interfered with comparative analysis of data. IMT protocols should be selected according to individual level of consciousness, cognitive capacity, and diagnosis. Training load should be adjusted according to treatment goals (muscle strength, muscle endurance, or both).
Conclusions
This sample included critically ill subjects undergoing mechanical ventilation with prolonged ICU length of stay who underwent IMT. IMT modalities investigated had no significant impacts on weaning time or successful weaning rates.
Footnotes
- Correspondence: Lígia dos Santos Roceto Ratti PT PhD, Vital Brasil Street, 251, University City, Campinas, São Paulo, Brazil 13083–888 E-mail: ligiasro{at}unicamp.br
This project was registered in the Brazilian Clinical Trial Registry, registration No. U1111-11563177.
The authors have disclosed no conflicts of interest.
Versions of this paper were presented by Dr Ratti at the European Respiratory Society International Congress 2018, held in Paris, France, September 15–19, 2018; and at the 23rd Brazilian Congress of Intensive Care, held in São Paulo, Brazil, November 29–December 1, 2018.
- Copyright © 2022 by Daedalus Enterprises