Abstract
BACKGROUND: Long-term oxygen therapy in COPD is usually supervised through home-care respiratory programs. Such programs often involve an intensive education intervention at the initiation of long-term oxygen therapy, followed by an extended follow-up period that aims toward home oxygen adherence. The objective of this study was to estimate the cost-effectiveness ratio of such a maintenance program.
METHODS: A simulation model was developed that compared 2 strategies after the intensive education intervention: (1) enrollment and (2) no enrollment in a maintenance program. The study population consisted of a hypothetical cohort of 200 patients (100 patients per group; mean age, 74 years; 45% men; mean FEV1 of 43% predicted value; and mean resting PaO2 while breathing air, 50 mm Hg). Effectiveness assumptions of the program were derived from a current literature review. The primary outcome was the ratio of the incremental cost of the program per quality-adjusted life-years gained. Only direct costs were considered; a health-care system perspective was adopted. Costs are reported in 2020 Canadian dollars (Can $).
RESULTS: Over a 5-year period, an extended home-visit program may prevent 9 deaths and provide an additional 39 years of life and 24 quality-adjusted life-years. Compared with usual care (ie, no enrollment in the maintenance program), the incremental cost-effectiveness ratio was Can $17,197 per quality-adjusted life-years gained. Sensitivity analyses demonstrated the robustness of the model. Only a reduction in adherence of 25% per year would increase the incremental cost-effectiveness ratio per quality-adjusted life-years beyond the threshold of Can $50,000 that is usually considered as acceptable from a health-care system perspective.
CONCLUSIONS: An extended home-visit program to maintain or improve adherence to long-term oxygen therapy in patients with COPD would most likely be cost-effective.
- long-term oxygen therapy
- home oxygen therapy
- COPD
- adherence
- cost
- cost-effectiveness analysis
- cost-utility analysis
- health economics
Introduction
Two randomized controlled trials of long-term oxygen therapy (LTOT) in COPD clearly demonstrated that low-flow domiciliary oxygen increases survival in patients with severe hypoxemia.1,2 A British study assigned subjects to receive 15 h/d of oxygen therapy (including hours of sleep) or no oxygen therapy at all,1 whereas an American trial randomized subjects to oxygen for either 12 h/d (nocturnal group) or 24 h/d (continuous group).2 The 2 studies included a total of 290 subjects and still represent the scientific base for the provision of LTOT in COPD around the world.3 Yet, home oxygen therapy comes in second place, only after hospitalizations, as the most expensive health-care expenditure associated with clinical care for COPD in developed countries.4
The indirect comparison of the results of these 2 trials suggests that the benefits of LTOT depend on the duration of daily exposure to oxygen.1,2 Unfortunately, adherence to home oxygen therapy is often suboptimal,5 which compromises the efficacy of the treatment. Predictors of adherence to oxygen therapy include supplemental education on home oxygen therapy by a health-care professional, smoking cessation, and the absence of adverse effects from oxygen treatment.6 Accordingly, careful patient selection, education about oxygen therapy, and close follow-up may be effective in maximizing adherence to oxygen7 and, consequently, in maximizing its potential benefits, including prolonged survival.8
In our institution, as in many other jurisdictions, LTOT is provided and supervised through a home-care respiratory program that comprises nurses and respiratory therapists. Newly admitted patients are delivered a 12-week educational intervention at the initiation of LTOT, followed by an extended teaching maintenance program that aims to improve and maintain patient adherence to home oxygen. The objective of this study was to estimate the cost-effectiveness ratio of this extended intervention.
QUICK LOOK
Current Knowledge
Long-term oxygen therapy improves survival in patients with COPD and severe daytime hypoxemia. Once long-term oxygen therapy is initiated, an intensive training period, including home visits by a specialist respiratory team, is considered as a standard of care for all patients to ensure adherence with therapy and to prepare patients for follow-up assessment. The cost-effectiveness of an extended home-visit program that aims at improving and maintaining patient adherence to home oxygen is uncertain.
What This Paper Contributes to Our Knowledge
In a cost-utility analysis based on a simulation model, we determined that, over a 5-year period, an extended home-visit program would prevent deaths and provide additional years of life. The incremental cost-utility ratio of such a program would not exceed the threshold that is usually considered as acceptable from a health-care system perspective. An extended home-visit program to maintain or improve adherence to long-term oxygen therapy in patients with COPD is most likely cost-effective.
Methods
Program Description and Clinical Pathway
Our respiratory home-care program is funded by the Quebec universal medical insurance plan. It delivers care (mainly LTOT and related services) and provides equipment (including oxygen concentrators) to patients with any chronic lung disease who meet the eligibility criteria for LTOT according to provincial terms of reference.9 To be admitted, patients must have an arterial 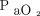 ≤ 55 mm Hg or
≤ 55 mm Hg or 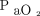 ≤ 59 mm Hg with clinical evidence of at least one of the following: right-ventricular hypertrophy, cor pulmonale, or hematocrit ≥ 55%.2 No patient is admitted on the basis of severe disability or on the basis of resting oxygen desaturation alone; arterial blood gas measurement, therefore, is mandatory. Most patients who receive LTOT within this program have COPD.
≤ 59 mm Hg with clinical evidence of at least one of the following: right-ventricular hypertrophy, cor pulmonale, or hematocrit ≥ 55%.2 No patient is admitted on the basis of severe disability or on the basis of resting oxygen desaturation alone; arterial blood gas measurement, therefore, is mandatory. Most patients who receive LTOT within this program have COPD.
Since 2009, patients are visited at home according to a prespecified schedule that can be adjusted to their needs. Once oxygen is prescribed, home visits occur every week for 6 weeks and every 2 weeks for an additional 6 weeks. This 12-week intensive teaching intervention is considered a standard of care for all patients to ensure adherence with therapy and to prepare patients for follow-up assessment.10 During this period, patients receive or revisit a self-management intervention based on the “Living Well with COPD” program (www.livingwellwithcopd.com, Accessed May 13, 2022), which includes a specific module about home oxygen.11 At a 3-month reassessment, clinical improvement to the point that home oxygen is not needed anymore may be demonstrated.12,13 Oxygen is then discontinued, and patients are discharged from the program. Patients who remain severely hypoxemic at the time of this re-evaluation then enter into an extended maintenance program that is the object of this cost-effectiveness analysis.
Those who were prescribed oxygen for ≥ 18 h/d, those with ≥ 4 exacerbations of COPD in the previous year, and those who demonstrated a limited ability to perceive that clinical deterioration will receive home visits from a nurse or a respiratory therapist in alternation every 12 weeks to ascertain clinical stability and to ensure their proper utilization of oxygen. During these 1-h visits, adherence to oxygen therapy and self-management strategies are reinforced. Clinical assessment, including clinical history, physical examination, and pulse oximetry is also performed. Symptoms or signs of clinical deterioration are reported to the attending or the treating physician who then takes action as needed. Home oxygen concentrator functioning is verified during the visits by the respiratory therapist. In addition, patients are encouraged to contact their nurse or respiratory therapist to report any technical problem with their oxygen concentrator or any new respiratory symptom that may prompt action. Those who do not meet the criteria to be visited every 12 weeks are visited every 6 months and receive the same intervention, including telephone calls as needed.
Model Overview
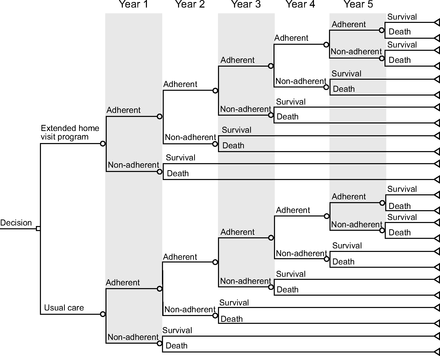
We constructed a decision tree for predicting the cost and the effect of the extended maintenance program to improve patients’ adherence to LTOT. The simulation model was developed with TreeAge Pro 2020 (TreeAge Software. Williamstown, Massachusetts). It compared 2 intervention strategies after the initial education period: (1) enrollment and (2) no enrollment in the extended maintenance program, with a time horizon of 5 years. Apart from enrollment (or no enrollment) in the home-visit program, all the patients were assumed to receive the same usual care (Fig. 1).
Decision tree for predicting the cost and the effect of an extended home-visit program to improve patient adherence to long-term oxygen therapy. Circle nodes are probability nodes. Triangle nodes are terminal nodes.
Clinical Inputs and Effectiveness Assumptions
Clinical inputs and effectiveness assumptions are shown in Table 1. The study population consisted of a hypothetical cohort of 200 patients with COPD (100 patients per group) treated at home with oxygen. We attributed to this cohort of patients the same characteristics as those that we reported in a study of adherence to oxygen therapy conducted in a “real” cohort of 115 subjects from our home-care program: mean age, 74 years; 45% men; mean FEV1 of 43% predicted value; and mean resting 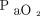 while breathing room air, 50 mm Hg.14 Adherence and non-adherence to home oxygen were defined as exposure to oxygen therapy for ≥18 h/d and <12 h/d, respectively. Adherent patients were attributed the same probability of survival as those who received continuous oxygen in the NOTT (Nocturnal Oxygen Therapy Trial),2 whereas non-adherent patients were given the same probability of survival as those who received nocturnal oxygen in the same trial. Because the NOTT investigators reported only 2-year survival rates (78% in the continuous oxygen therapy group and 59% in the nocturnal oxygen therapy group), we estimated, from exponential smoothing approximation, 5-year survival to be 59% and 21% in the continuous oxygen group and the nocturnal oxygen group, respectively.15
while breathing room air, 50 mm Hg.14 Adherence and non-adherence to home oxygen were defined as exposure to oxygen therapy for ≥18 h/d and <12 h/d, respectively. Adherent patients were attributed the same probability of survival as those who received continuous oxygen in the NOTT (Nocturnal Oxygen Therapy Trial),2 whereas non-adherent patients were given the same probability of survival as those who received nocturnal oxygen in the same trial. Because the NOTT investigators reported only 2-year survival rates (78% in the continuous oxygen therapy group and 59% in the nocturnal oxygen therapy group), we estimated, from exponential smoothing approximation, 5-year survival to be 59% and 21% in the continuous oxygen group and the nocturnal oxygen group, respectively.15
Effectiveness Assumption, Outcome Data, and Cost Data
We also used the results of our study of adherence to LTOT to estimate the proportion of adherent patients among our hypothetical cohort of patients.14 In this study of 115 subjects with oxygen-dependent COPD, most were prescribed oxygen for ≥18 h/d. Daily exposure to oxygen was objectively measured from the concentrator’s counter clock. All received an education program as described above and were admitted to the extended maintenance program. As per our definition of adherence to LTOT, 60% of the subjects were adherent. The assumption of effectiveness of the follow-up program was derived from a British study that assessed the improvement of patient adherence with LTOT after formal assessment and training.7 In this study, 82% of the subjects of the education group were using their concentrator for >15 h compared with 44% for the control group, which suggests that the program had a relative effectiveness of 86% after 6 months ([82% – 44%]/44%). By applying these results to those of our own study of adherence to oxygen in COPD,14 we estimated the adherence of the control group not exposed to the extended teaching program. In the primary analysis, we assumed that the relative effectiveness was constant over time.
Health Utility Values
The health outcome used in the primary analysis was quality adjusted life-years. To adjust the life year gained to quality-adjusted life-years (QALY), we applied a utility score of 0.60 for each year gained, hence reducing by 40% the total values of year gained in perfect health. This value was obtained from a cross-sectional study that was specifically designed to determine utility scores in patients with COPD who received LTOT.16 The effect of COPD exacerbations or COPD-related hospitalizations on QALY was not considered in the analysis because there currently is no evidence to suggest that LTOT reduces the rate of exacerbations in COPD.
Costs
A health-care system perspective was adopted. Only direct costs were considered. Because the only difference between the 2 interventions was the cost of the program, we did not include the cost of acquiring and running the oxygen concentrator. The cost of the educational program was assumed to consist of one home visit by the nurse every 12 weeks per year for our time horizon analysis. We also assumed one telephone call per patient every 2 months. Nurse travel costs were assumed to be Can $54 per unit. This would include travel costs and 30 min of travel time. We used the Quebec Nursing agreement salary to extract the hourly rate of a level-9 seniority nurse and accounted the indirect benefit on top of the hourly salary. All costs are reported in 2020 Canadian dollars.
Analysis
The incremental cost-effectiveness ratio was calculated as the following:
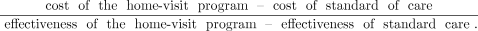
We calculated incremental cost-effectiveness ratio for survival (death avoided), life years gained, and QALY. To test the robustness of the results, deterministic univariate (Tornado) and probabilistic multivariate sensitivity analyses were performed on the main model variables by varying clinical parameters by ±20% from their baseline point estimate (Table 1).17 Threshold analyses were also performed by considering waning of the program effectiveness (ie, a decrease in adherence to oxygen therapy translating in a reduction in the number of hours of exposure to oxygen per day) over time and by varying program costs to identify of a specific value at which the incremental cost-effectiveness ratio per QALY would exceed the acceptable incremental cost-effectiveness ratio threshold.17 In the multivariate sensitivity analysis, the results of 1,000 simulations are summarized by using a cost-effectiveness acceptability curve that reflects the proportion of the results that are considered cost-effective in relation to a given cost-effectiveness threshold.18 We determined a priori that a willingness-to-pay threshold of Can $50,000 per QALY would be acceptable from the health-care system perspective.19 Given that the time horizon for the analysis was 5 years, a 3% discounting rate of the costs or the effects was applied.17
Results
Main Analyses: Incremental Cost-Effectiveness Ratios
The total incremental costs and quality-adjusted life-years are summarized in Tables 2-4, along with the incremental cost-effectiveness ratio. Over a 5-year period, the program would prevent 9 deaths and provide an additional 39 years of life and 24 QALY compared to standard care at an acceptable incremental cost. The incremental cost-effectiveness ratio per quality-adjusted life-years gained (Can $17,197) was lower than the incremental cost-effectiveness ratio threshold of Can $50,000 that we determined a priori.
Incremental Cost-Effectiveness Ratios per Deaths Avoided
Incremental Cost-Effectiveness Ratio per Life Year Gained
Incremental Cost-Effectiveness Ratio per Quality-Adjusted Life-Years Gained
Sensitivity Analyses
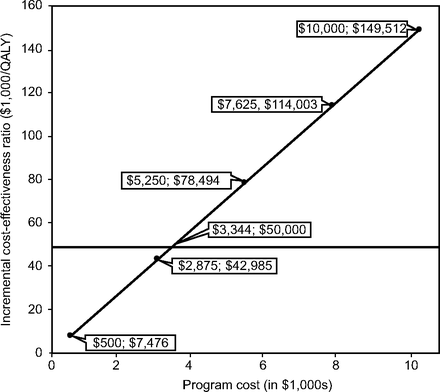
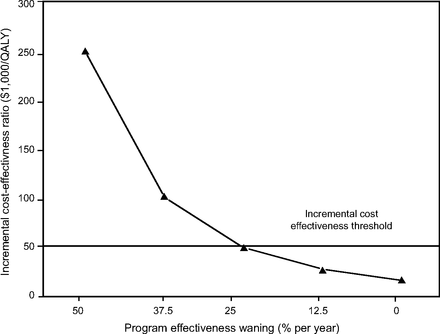
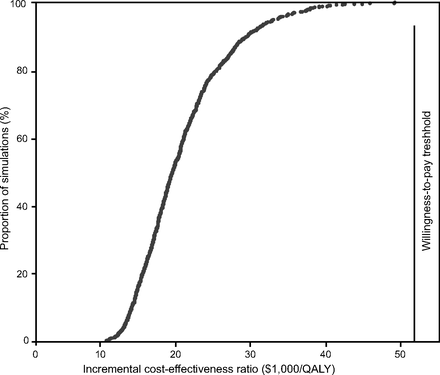
The results of the Tornado sensitivity analyses for the incremental cost-effectiveness ratios are presented in Table 5. Except for waning of efficacy, all the incremental cost-effectiveness ratio for the extended home-visit program were less than Can $50,000 per QALY, which supports the robustness of the baseline analysis. Increasing the cost of the program to Can $2,000 per patient per year would not have a significant impact on the cost-effectiveness of the program. It is only if the program cost was higher than Can $3,344 per patient per year that its incremental cost-effectiveness ratio would be higher than the willingness-to-pay threshold of Can $50,000 per QALY (Fig. 2). Threshold analyses indicated that, beyond a waning of 25% per year, the education program would not be cost-effective if Can $50,000 per quality-adjusted life-years is used as a cutoff (Fig. 3). In the multivariate sensitivity analysis, the median and maximum incremental cost-effectiveness ratios were Can $18,574 per QALY and Can $48,132 per QALY, respectively (Fig. 4).
Univariate Sensitivity Analyses: Incremental Cost-Effectiveness Ratios After Varying Clinical Parameters From Baseline Point Estimate
The impact of the cost of the home-visit program on its incremental cost-effectiveness ratio.
Impact of waning of effectiveness of the home-visit program on its incremental cost-effectiveness ratio.
A cost-effectiveness acceptability curve that indicates that 100% of the 1,000 simulations are cost-effective in relation to the cost-effectiveness threshold of Can $50,000 per quality-adjusted life-years.
Discussion
In this cost-effectiveness study of an extended home-visit program to maintain or improve adherence to LTOT in patients with COPD, we determined that the incremental cost-effectiveness ratio per QALY gained (Can $17,197) with the program is much lower than the incremental cost-effectiveness ratio threshold of Can $50,000 that would now be considered as conservative from a health-care system perspective.20 Sensitivity analyses supported the robustness of the main analysis. We estimated that the annual cost per patient of our program is Can $1,150. The program would remain cost-effective, even if its cost was increased to Can $2,000 per patient per year.
Home oxygen therapy for patients with COPD is expensive, as indicated by the recent Continuing to Confront COPD International Patient Survey.4 This population-based, cross-sectional survey aimed at estimating the prevalence and burden of COPD across 12 countries around the world. A total of 4,343 respondents were included. The annual societal cost per patient who encompassed both direct and indirect costs from loss of productivity ranged from Can $1,721 in Russia to Can $30,826 in the United States. Although this survey targeted patients with COPD of any severity, in-patient hospitalizations and home oxygen therapy were the key drivers of direct costs in 5 and 3 countries, respectively. Home oxygen therapy consistently accounted for the largest individual direct costs in Brazil (28%), Russia (33%), France (30%), and Italy (40%). Our study indicated that an extended home-visit program would account for only a small proportion of the costs incurred by LTOT.
Beyond the mere determination of the cost of LTOT, a full economic analysis requires that both the costs and the effectiveness of competing strategies be compared.21 In this regard, we are aware of 2 cost-effectiveness studies of LTOT published since 2009.22,23 In these 2 studies, cost-utility analyses were conducted based on Markov probabilistic models. The main objective of both studies was to estimate the incremental cost-effectiveness ratio for LTOT relative to no oxygen therapy at all (ie, “usual care” or “do-nothing strategy”). According to the study by Oba,22 cost data were limited to the Medicare reimbursement rate for oxygen therapy, stationary oxygen equipment with or without a portable system and electricity to run the concentrator. The total cost was estimated to be $2,380 per year (reported in 2007 United States dollars).22 According to the study by Chandra et al,23 the average annual cost per patient for LTOT was Can $2,261 (reported in 2006 Canadian dollars) and included home assessment, 24-h emergency service, maintenance and repair, training and education, oxygen supply system, and disposables. In both studies, LTOT was found to be cost-effective (Can $23,807 per QALY during a 3-year horizon and Can $16,124 per quality-adjusted life-years during a 5-year horizon in the study by Oba,22 and Can $38,993 per QALY during a life-long horizon in the study by Chandra et al23). Although these studies provided interesting information, their results could hardly inform decisions on the opportunity to offer or not to offer LTOT because LTOT has been considered as a standard of care for more than 40 years.1,2 Now, the issue is not to decide whether LTOT should be provided but rather to decide how it can be delivered most efficiently.
Our study compared 2 intervention strategies in subjects treated at home with supplemental oxygen. The question that we addressed was whether long-term supportive efforts to maintain home oxygen therapy adherence in subjects with COPD and severe hypoxemia are cost-effective. The costs involved were limited to those incurred from the home visits and telephone calls. By fostering adherence to home oxygen, our analysis suggested that the maintenance program is cost-effective in terms of quality-adjusted life-years gained. This contrasts with the results of a detailed economic analysis of a randomized trial of an intensive telehealth program for subjects with COPD treated at home with LTOT.24 In this study conducted in Spain, the incremental cost-effectiveness ratio of the program was €223,726 per QALY. However, subgroup analyses indicated that the program may be cost-effective in patients without comorbidity.
Our study had limitations. The most obvious was that it was based on modeling. Assumptions of effectiveness of our extended program came from a 6-month observational study that compared formal assessment and training to receiving LTOT with no training at all.7 Nevertheless, more recently, we demonstrated that long-term adherence to LTOT may be maintained, especially when oxygen is prescribed for 18 h/d (as opposed to 24 h/d).14 Costs were limited to those of nurses and respiratory therapists involved in the program. Although hospitalizations are the main driver of cost in severe COPD,4 there currently is no evidence that LTOT decreases the rate of exacerbations and hospitalizations in COPD. Another assumption is that gains in quality-adjusted life-years are only from improved survival. Few diseases are associated with worse quality of life than oxygen-dependent COPD16 and whether LTOT truly improves quality of life in end-stage oxygen-dependent COPD is doubtful.
Conclusions
Questions and concerns with regard to the cost-effectiveness of LTOT were raised, and suggestions as to how to provide continued care to patients who receive LTOT to increase adherence were proposed in this Journal 20 years ago: “Given the evidence now being reported that adherence in using home oxygen as prescribed may well be much lower than originally believed, the time is probably right to revisit the role played by home oxygen providers in determining continuing need through the performance of periodic reassessments. Such reassessments, if designed according to prescribed and validated protocols and conducted by home respiratory therapists under orders of the prescribing physician, would (...) help ensure that those needing and using home oxygen would continue to receive the benefit.”25 Our extended home-visit program fulfills these conditions. The results of our cost-effectiveness analysis are reassuring: an extended home-visit program to maintain or improve adherence to LTOT in patients with COPD is cost-effective.
Footnotes
- Correspondence: Yves Lacasse MD, Centre de Pneumologie, Institut Universitaire de Cardiologie et de Pneumologie de Québec – Université Laval, 2725, Chemin Sainte-Foy, Québec, G1V 4G5, Canada. E-mail: Yves.Lacasse{at}med.ulaval.ca
See the Related Editorial on Page 1211
Financial support was provided by Groupe de Recherche en Santé Respiratoire de l’Université Laval.
The authors have disclosed no conflicts of interest.
- Copyright © 2022 by Daedalus Enterprises