Abstract
BACKGROUND: Chronic hypercapnic respiratory failure is associated with high mortality. Although previous work has demonstrated a mortality improvement with high-intensity noninvasive ventilation in COPD, it is unclear whether a PCO2 reduction strategy is associated with improved outcomes in other populations of chronic hypercapnia.
METHODS: The objective of this study was to investigate the association between PCO2 reduction (by using transcutaneous PCO2 as an estimate for PaCO2 and survival in a broad population of individuals treated with noninvasive ventilation for chronic hypercapnia. We hypothesized that reductions in PCO2 would be associated with improved survival. Therefore, we performed a cohort study of all the subjects evaluated from February 2012 to January 2021 for noninvasive ventilation initiation and/or optimization due to chronic hypercapnia at a home ventilation clinic in an academic center. We used multivariable Cox proportional hazard models with time-varying coefficients and PCO2 as a time-varying covariate to test the association between PCO2 and all-cause mortality and when adjusting for known cofounders.
RESULTS: The mean ± SD age of 337 subjects was 57 ± 16 years, 37% women, and 85% white. In a univariate analysis, survival probability increased with reductions in PCO2 to < 50 mm Hg after 90 d, and these remained significant after adjusting for age, sex, race, body mass index, diagnosis, Charlson comorbidity index, and baseline PCO2. In the multivariable analysis, the subjects who had a PaCO2 < 50 mm Hg had a reduced mortality risk of 94% between 90 and 179 d (hazard ratio [HR] 0.06, 95% CI 0.01–0.50), 69% between 180 and 364 d (HR 0.31, 95% CI 0.12–0.79), and 73% for 365–730 d (HR 0.27, 95% CI 0.13–0.56).
CONCLUSIONS: Reduction in PCO2 from baseline for subjects with chronic hypercapnia treated with noninvasive ventilation was associated with improved survival. Management strategies should target the greatest attainable reductions in PCO2.
- noninvasive ventilation
- hypercapnia
- respiratory insufficiency
- mortality
- amyotrophic lateral sclerosis
- neuromuscular diseases
- chronic obstructive pulmonary disease
Introduction
Elevated PCO2 is associated with increased mortality and morbidity.1,2 Although the causes are likely multifactorial, the association could be indirectly related to the severity of underlying disease (irrespective of the etiology) or directly through several pathophysiologic mechanisms. For example, elevated PCO2 may hinder both skeletal muscle repair and host defenses against bacterial infections, which leads to increased mortality.3,4 In addition, overloaded body tissue stores of CO2 can result in a rapid, potentially intolerable further  increments in response to an increase in CO2 production or reduction in CO2 removal.5
increments in response to an increase in CO2 production or reduction in CO2 removal.5
In the setting of chronic alveolar hypoventilation, noninvasive ventilation (NIV) can offload respiratory muscles while increasing alveolar ventilation to improve CO2 clearance and reduce excess total body CO2 stores.5 NIV has been shown to improve survival in many conditions, including COPD,6,-,8 obesity-hypoventilation syndrome,9,-,11 and amyotrophic lateral sclerosis (ALS).12 Although the importance of out-patient NIV therapy is becoming increasingly recognized,13,14 there remain few data with regard to optimal treatment strategies and targets.
Transcutaneous carbon dioxide (PtcCO2) monitors are noninvasive devices that provide a real-time proxy for 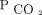 levels.15 Their reliability and portability provide clinicians with a convenient, practical tool to follow
levels.15 Their reliability and portability provide clinicians with a convenient, practical tool to follow 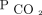 . Aarrestad et al16 reported that PtcCO2 monitoring reflects
. Aarrestad et al16 reported that PtcCO2 monitoring reflects 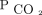 in subjects who are stable and receiving NIV,17 with limits of agreements within the proposed ranges.18,19
in subjects who are stable and receiving NIV,17 with limits of agreements within the proposed ranges.18,19
The purpose of this study was to determine whether reductions in  (by using PtcCO2 as an estimate for
(by using PtcCO2 as an estimate for 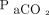 ) achieved through NIV are associated with improved survival in a sample of subjects with mixed etiologies of chronic hypercapnia. We hypothesized that reducing
) achieved through NIV are associated with improved survival in a sample of subjects with mixed etiologies of chronic hypercapnia. We hypothesized that reducing  through NIV is associated with improved survival and that the survival benefit is dose-dependent based on the magnitude of
through NIV is associated with improved survival and that the survival benefit is dose-dependent based on the magnitude of 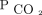 decrease from baseline.
decrease from baseline.
QUICK LOOK
Current Knowledge
Chronic compensated hypercapnia is associated with increased mortality and morbidity. In the setting of chronic alveolar hypoventilation, noninvasive ventilation can offload respiratory muscles while increasing alveolar ventilation and reducing excess total body PCO2 stores.
What This Paper Contributes to Our Knowledge
The reduction in PCO2 levels by using noninvasive ventilation is associated with improved survival in chronic hypercapnic respiratory failure. Larger reductions in PCO2 had a stronger association with survival. Reductions of PCO2 to levels < 50 mm Hg were achieved in most subjects.
Methods
We conducted a retrospective cohort study of adult subjects (≥18 years) with chronic hypercapnic respiratory failure of any cause assessed for NIV initiation or optimization by the assisted ventilation clinic at a reference academic center. The institutional review board approved this study (HUM00162425) and waived the informed consent requirement for data collection. By using existing clinic patient databases, we identified all patients with a first-time encounter with the assisted ventilation clinic between February 2012 and January 2021. The first encounter was defined as either the first out-patient clinic visit or an in-patient encounter in which the assisted ventilation clinic team was consulted for the initiation of NIV.
We included subjects with chronic hypercapnia at the first encounter who were initiated or maintained on NIV. Chronic hypercapnia was defined as 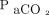 > 45 mm Hg with a pH > 7.35 in the in-patient setting, or PtcCO2 > 45 mm Hg by using PtcCO2 monitoring in a stable state as an out-patient. We excluded patients who required invasive mechanical ventilation (patients who were tracheostomized) at the first visit, those who were not initiated on NIV, and patients lost to follow-up.
> 45 mm Hg with a pH > 7.35 in the in-patient setting, or PtcCO2 > 45 mm Hg by using PtcCO2 monitoring in a stable state as an out-patient. We excluded patients who required invasive mechanical ventilation (patients who were tracheostomized) at the first visit, those who were not initiated on NIV, and patients lost to follow-up.
Follow-up 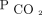 measurements over a 2-year period were performed by using PtcCO2 monitoring. We used electronic medical record chart review to identify demographic characteristics, comorbidities, and clinical data. All out-patient visits included a PtcCO2 reading while the subject was at rest, breathing spontaneously without assistance unless the clinical status required continuous mechanical ventilation. PtcCO2 measurements were performed by using a SenTec Digital Monitor (SenTec AG, Therwil, Switzerland). The PtcCO2 sensor was applied to the subject’s forehead at 42°C for at least 5–10 min, and the value at steady state was recorded.
measurements over a 2-year period were performed by using PtcCO2 monitoring. We used electronic medical record chart review to identify demographic characteristics, comorbidities, and clinical data. All out-patient visits included a PtcCO2 reading while the subject was at rest, breathing spontaneously without assistance unless the clinical status required continuous mechanical ventilation. PtcCO2 measurements were performed by using a SenTec Digital Monitor (SenTec AG, Therwil, Switzerland). The PtcCO2 sensor was applied to the subject’s forehead at 42°C for at least 5–10 min, and the value at steady state was recorded.
At each clinic visit, assessments were made by a pulmonary clinician and respiratory clinician. Based on PtcCO2 levels and device downloads, recommendations were made to continue current management strategies, adjust ventilator settings, or increase hours of use. For the subjects who had no change or had an increase in PtcCO2 from baseline during the follow-up period, the clinical notes were reviewed to determine the reasons for the lack of PtcCO2 improvement.
The primary outcome was all-cause mortality within 2 years of the initial encounter. We determined the vital status by reviewing the electronic medical record, funeral home web sites, and online obituaries. All the subjects were followed up from the date of the first encounter to death, the last visit, or August 30, 2021, whichever came first, with a maximum follow-up time of 730 d. Subjects were considered lost to follow-up when their status within the past 6 months of the censoring date was unknown.
We described baseline characteristics by using means ± SDs or medians (interquartile ranges) for continuous variables and as counts (percentage) for categorical variables. We used uni- and multivariable Cox proportional hazard models. To minimize immortal time bias,20 we included time-varying 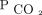 levels in the Cox proportional hazards models. To determine
levels in the Cox proportional hazards models. To determine 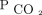 associations at different time intervals, we used time-varying coefficients of
associations at different time intervals, we used time-varying coefficients of 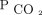 in the Cox proportional hazards models.21
in the Cox proportional hazards models.21
For the multivariable models, we adjusted for potential confounders that were associated with disease progression, a need for NIV, and overall survival in chronic respiratory failure, such as primary diagnosis for NIV indication, age, sex, race, body mass index, Charlson comorbidity index, and baseline 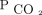 . The primary diagnosis was considered the primary reason for chronic respiratory failure. We examined the proportional hazard assumption of
. The primary diagnosis was considered the primary reason for chronic respiratory failure. We examined the proportional hazard assumption of 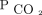 within each time interval through Schoenfeld residual plots.22,23
within each time interval through Schoenfeld residual plots.22,23
We performed subgroup analyses by using the aforementioned approach to examine associations with mortality, including subjects with ALS versus subjects without ALS and those with both baseline  and PtcCO2 versus baseline PtcCO2 alone. Missing data were handled by using multiple imputation. We considered all P values of <.05 as statistically significant. We performed all analyses by using Stata version 15.0 (StataCorp, College Station, Texas).
and PtcCO2 versus baseline PtcCO2 alone. Missing data were handled by using multiple imputation. We considered all P values of <.05 as statistically significant. We performed all analyses by using Stata version 15.0 (StataCorp, College Station, Texas).
Results
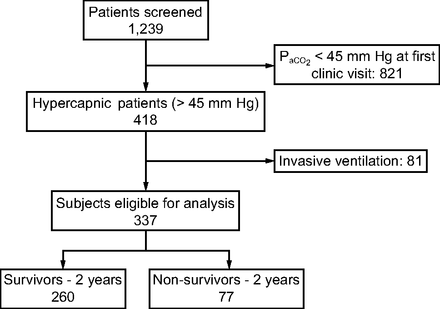
We identified 337 unique subjects who were evaluated at the assisted ventilation clinic for NIV initiation or optimization with baseline hypercapnia (Table 1). We excluded 81 patients who were receiving invasive mechanical ventilation at the time of the first visit (Fig. 1). The mean ± SD age was 57 ± 16 y, 125 (37%) were women, and 285 (85%) were white. The most common causes of chronic respiratory failure were neuromuscular diseases (NMD) other than ALS and restrictive thoracic disorders (41%), followed by ALS (16%), obesity-hypoventilation syndrome (14%), spinal cord injury (12%), and COPD (12%). Additional diagnoses (central congenital hypoventilation, pleural disease, cystic fibrosis, bronchiectasis) were categorized as other (5%). The mean ± SD baseline 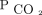 was 57 ± 10 mm Hg, with 40% ranging between 50 and 59 mm Hg. Most initial encounters occurred in the out-patient setting (78%), with 44% of the cohort already using NIV on referral to the assisted ventilation clinic. The median (interquartile range) follow-up time was 730 (600–730) d. With regard to missing data, we had one subject only in which the body mass index could not be calculated (height was missing throughout the chart).
was 57 ± 10 mm Hg, with 40% ranging between 50 and 59 mm Hg. Most initial encounters occurred in the out-patient setting (78%), with 44% of the cohort already using NIV on referral to the assisted ventilation clinic. The median (interquartile range) follow-up time was 730 (600–730) d. With regard to missing data, we had one subject only in which the body mass index could not be calculated (height was missing throughout the chart).
Flow chart.
Cohort Characteristics (N = 337)
Among the different diagnostic subgroups, the mean ± SD baseline  were highest in the subjects with COPD (66 ± 13 mm Hg) and lowest in those with ALS (51 ± 6 mm Hg). Subjects initially encountered as in-patients had higher
were highest in the subjects with COPD (66 ± 13 mm Hg) and lowest in those with ALS (51 ± 6 mm Hg). Subjects initially encountered as in-patients had higher 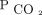 levels than those encountered as out-patients. Irrespective of the diagnosis, the mean
levels than those encountered as out-patients. Irrespective of the diagnosis, the mean 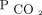 was reduced for subjects in all the subgroups at 0–90 d, 90–180 d, 180–365 d, 365–540 d, and 540–730 d after the first encounter. The mean
was reduced for subjects in all the subgroups at 0–90 d, 90–180 d, 180–365 d, 365–540 d, and 540–730 d after the first encounter. The mean 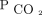 was < 50 mm Hg for surviving subjects in all primary diagnosis subgroups across both initial assessment locations at 1 and 2 years except for COPD (Table 2).
was < 50 mm Hg for surviving subjects in all primary diagnosis subgroups across both initial assessment locations at 1 and 2 years except for COPD (Table 2).
PCO2 Averages by Time and Group
We found a 23% all-cause mortality within 2 years of the first evaluation at the assisted ventilation clinic. In the univariate analysis, we found significant associations between mortality and the diagnoses of ALS (hazard ratio [HR] 10.60, 95% CI 4.60–13.30) and COPD (HR 4.37, 95% CI 1.68–6.34). Female sex, higher age, body mass index ≥ 30 kg/m2, and higher Charlson comorbidity index were associated with mortality.
Survival probabilities increased after 90 d based on the percent reduction in  from baseline (Fig. 2). In the multivariate analysis, we found a 92% reduction in mortality between 180 and 364 d (HR 0.08, 95% CI 0.01–0.64) for the subjects with a reduction of >20% from the baseline
from baseline (Fig. 2). In the multivariate analysis, we found a 92% reduction in mortality between 180 and 364 d (HR 0.08, 95% CI 0.01–0.64) for the subjects with a reduction of >20% from the baseline  . We did not find statistically significant differences in the other deciles of
. We did not find statistically significant differences in the other deciles of 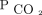 reduction or at < 180 d. However, after 365 d, we found a 77% reduction in mortality in subjects who had a reduction in
reduction or at < 180 d. However, after 365 d, we found a 77% reduction in mortality in subjects who had a reduction in 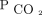 between 10 and 19.9% (HR 0.23, 95% CI 0.08–0.65) and a 76% reduction in those who had a reduction in
between 10 and 19.9% (HR 0.23, 95% CI 0.08–0.65) and a 76% reduction in those who had a reduction in 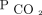 > 20% (HR 0.24, 95% CI 0.09 – 0.62) (Table 3).
> 20% (HR 0.24, 95% CI 0.09 – 0.62) (Table 3).
Two-year survival by the percentage of 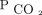 change for the entire cohort of subjects who were hypercapnic. The model was adjusted for age, sex, body mass index, race, Charlson comorbidity index, primary diagnosis, and initial
change for the entire cohort of subjects who were hypercapnic. The model was adjusted for age, sex, body mass index, race, Charlson comorbidity index, primary diagnosis, and initial 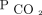 . Likelihood ratio test of equality, P ≤ .001.
. Likelihood ratio test of equality, P ≤ .001.
Results of a 2-y Survival Analysis Adjusted for Percent Change in  (N = 337)
(N = 337)
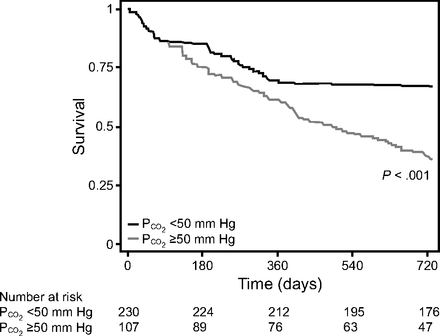
Similarly, absolute values of 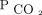 < 50 mm Hg after 90 d were associated with improved survival (Fig. 3). After adjusting for age, sex, race, body mass index, primary diagnosis, Charlson comorbidity index, and baseline
< 50 mm Hg after 90 d were associated with improved survival (Fig. 3). After adjusting for age, sex, race, body mass index, primary diagnosis, Charlson comorbidity index, and baseline 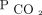 , there remained an increase in survival probability after 90 d for the subjects who attained
, there remained an increase in survival probability after 90 d for the subjects who attained 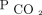 < 50 mm Hg. In the multivariable analysis, we found a 94% reduction in mortality between 90 and 179 d (HR 0.06, 95% CI 0.01–0.50), a 69% reduction in mortality between 180 and 364 d (HR 0.31, 95% CI 0.12–0.79), and a 73% reduction in mortality between 365 and 730 d (HR 0.27, 95% CI 0.13 – 0.56) for the subjects who attained
< 50 mm Hg. In the multivariable analysis, we found a 94% reduction in mortality between 90 and 179 d (HR 0.06, 95% CI 0.01–0.50), a 69% reduction in mortality between 180 and 364 d (HR 0.31, 95% CI 0.12–0.79), and a 73% reduction in mortality between 365 and 730 d (HR 0.27, 95% CI 0.13 – 0.56) for the subjects who attained 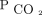 < 50 mm Hg (Table 4).
< 50 mm Hg (Table 4).
Two-year survival by absolute  higher on < 50 mm Hg for the entire cohort of subjects who were hypercapnic. The model was adjusted for age, sex, body mass index, race, Charlson comorbidity index, primary diagnosis, and initial
higher on < 50 mm Hg for the entire cohort of subjects who were hypercapnic. The model was adjusted for age, sex, body mass index, race, Charlson comorbidity index, primary diagnosis, and initial 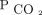 . Likelihood ratio test of equality, P ≤ .001.
. Likelihood ratio test of equality, P ≤ .001.
Results of 2-y Survival Analysis Adjusted for Absolute PCO2 > 50 mm Hg or < 50 mm Hg (N = 337)
To examine the potential effect modification of ALS, a disease with high short-term mortality, we performed a subgroup analysis that considered subjects with (Supplementary Figs. 1 and 2 and Supplementary Table 1) and without ALS (Supplementary Figs. 3 and 4) (see the supplementary materials at http://www.rcjournal.com). Due to the imperfect correlation between PtcCO2 and  , we analyzed survival by considering those who had only PtcCO2 measurements at baseline (Supplementary Fig. 5 [see the supplementary materials at http://www.rcjournal.com]). Similarly, we analyzed survival subjects who were NIV naive only (Supplementary Table 2 and Supplementary Fig. 6 [see the supplementary materials at http://www.rcjournal.com]). All subgroup analyses yielded similar results to our primary analysis. Forty-two subjects were unable to achieve any reduction in
, we analyzed survival by considering those who had only PtcCO2 measurements at baseline (Supplementary Fig. 5 [see the supplementary materials at http://www.rcjournal.com]). Similarly, we analyzed survival subjects who were NIV naive only (Supplementary Table 2 and Supplementary Fig. 6 [see the supplementary materials at http://www.rcjournal.com]). All subgroup analyses yielded similar results to our primary analysis. Forty-two subjects were unable to achieve any reduction in  from baseline in 6–12 months. Of these 42 subjects, 12 (29%) were non-adherent with therapy, defined as consistently < 4 h of daily usage. The remaining 30 subjects (71%) were still unable to achieve a reduction in
from baseline in 6–12 months. Of these 42 subjects, 12 (29%) were non-adherent with therapy, defined as consistently < 4 h of daily usage. The remaining 30 subjects (71%) were still unable to achieve a reduction in 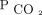 , despite > 4 h of consistent daily usage.
, despite > 4 h of consistent daily usage.
Discussion
In this single-center, retrospective study of subjects with chronic hypercapnic respiratory failure, we found a strong correlation between reduction in diurnal 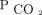 by means of NIV and 2-year mortality. A reduction in
by means of NIV and 2-year mortality. A reduction in 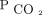 to <50 mm Hg by 3–6 months was associated with improved survival. In addition, increasing the percent reduction in
to <50 mm Hg by 3–6 months was associated with improved survival. In addition, increasing the percent reduction in  from baseline was also associated with improved survival. Baseline hypercapnia can be a marker for more-severe cardiovascular-pulmonary or multi-organ system disease. Although the cause-effect relationship between
from baseline was also associated with improved survival. Baseline hypercapnia can be a marker for more-severe cardiovascular-pulmonary or multi-organ system disease. Although the cause-effect relationship between  reduction and 2-year mortality cannot be ascertained from this retrospective study, there are compelling reasons to suspect that improving diurnal
reduction and 2-year mortality cannot be ascertained from this retrospective study, there are compelling reasons to suspect that improving diurnal 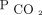 in a dose-response manner could explain the prolonged survival.
in a dose-response manner could explain the prolonged survival.
Elevated 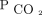 directly impairs alveolar fluid reabsorption,24 epithelial cell regeneration,25 cytokine expression,26 and phagocytosis,27 independent of extracellular pH. In mice infected with Pseudomonas aeruginosa and Influenza A, hypercapnia was associated with increased mortality, independent from blood pH.4,28 The researchers attributed these findings to the effects of hypercapnia on neutrophil and macrophage activity, which suggest that patients with chronic hypercapnia may be predisposed to developing life-threatening bacterial and viral infections. The concept of hypercapnia induced immune dysfunction is supported by observational studies of community-acquired pneumonia that demonstrated an association between hypercapnia and increased ICU admission, intubation, and mortality.29,30
directly impairs alveolar fluid reabsorption,24 epithelial cell regeneration,25 cytokine expression,26 and phagocytosis,27 independent of extracellular pH. In mice infected with Pseudomonas aeruginosa and Influenza A, hypercapnia was associated with increased mortality, independent from blood pH.4,28 The researchers attributed these findings to the effects of hypercapnia on neutrophil and macrophage activity, which suggest that patients with chronic hypercapnia may be predisposed to developing life-threatening bacterial and viral infections. The concept of hypercapnia induced immune dysfunction is supported by observational studies of community-acquired pneumonia that demonstrated an association between hypercapnia and increased ICU admission, intubation, and mortality.29,30
In addition to its effects on host defenses, elevated 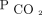 has harmful effects on the respiratory pump. Hypercapnia, in both animal and human studies, has been shown to have direct effects on skeletal muscle function. Hypercapnia directly leads to catabolic muscle wasting while also impairing muscle regeneration in respiratory and non-respiratory skeletal muscles.3,31 Patients with conditions that lead to alveolar hypoventilation may develop functional neuromuscular weakness. Adverse effects on brain function have been identified.32,-,34 In addition, chronic hypercapnia increases total body tissue stores of
has harmful effects on the respiratory pump. Hypercapnia, in both animal and human studies, has been shown to have direct effects on skeletal muscle function. Hypercapnia directly leads to catabolic muscle wasting while also impairing muscle regeneration in respiratory and non-respiratory skeletal muscles.3,31 Patients with conditions that lead to alveolar hypoventilation may develop functional neuromuscular weakness. Adverse effects on brain function have been identified.32,-,34 In addition, chronic hypercapnia increases total body tissue stores of 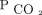 , which renders patients vulnerable to acid/base and cardiorespiratory decompensation in the event of impairments in
, which renders patients vulnerable to acid/base and cardiorespiratory decompensation in the event of impairments in 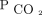 removal or modest acute increases in
removal or modest acute increases in 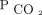 production as might occur during a febrile illness.5
production as might occur during a febrile illness.5
Our study showed that, although  could be reduced within the first 3 months after the first visit, its impact on survival became evident with sustained
could be reduced within the first 3 months after the first visit, its impact on survival became evident with sustained  reductions. This was seen in both the analyses of percent
reductions. This was seen in both the analyses of percent 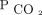 reduction as well as the
reduction as well as the 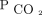 reduction to < 50 mm Hg. These findings may simply be due to the infrequency of deaths within the first 3 months of evaluation. However, this greater survival benefit over time may also represent the time needed to affect host defenses and skeletal muscle function. Future studies that look at these factors in response to
reduction to < 50 mm Hg. These findings may simply be due to the infrequency of deaths within the first 3 months of evaluation. However, this greater survival benefit over time may also represent the time needed to affect host defenses and skeletal muscle function. Future studies that look at these factors in response to 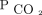 reduction in humans are warranted. Analysis of our findings suggests that future studies in hypercapnic respiratory failure should aim to explore the reduction in
reduction in humans are warranted. Analysis of our findings suggests that future studies in hypercapnic respiratory failure should aim to explore the reduction in 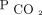 and maintain the
and maintain the 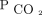 reduction over time.
reduction over time.
Wilson et al2 demonstrated that, in hospitalized patients with compensated hypercapnia, higher levels of  were associated with increased mortality even when adjusted for severity of illness. In contrast to our study, Wilson et al2 studied a population that mostly comprised subjects with obstructive sleep apnea, COPD, and heart failure, whereas only a minority had NMD. In our study, 57% of the population had a primary neuromuscular diagnosis as the cause for hypercapnic respiratory failure. Given the poor prognosis in specific neuromuscular disorders, for example, ALS, we performed sensitivity analyses excluding patients with ALS in which we found a consistent signal for survival benefit in those who decreased
were associated with increased mortality even when adjusted for severity of illness. In contrast to our study, Wilson et al2 studied a population that mostly comprised subjects with obstructive sleep apnea, COPD, and heart failure, whereas only a minority had NMD. In our study, 57% of the population had a primary neuromuscular diagnosis as the cause for hypercapnic respiratory failure. Given the poor prognosis in specific neuromuscular disorders, for example, ALS, we performed sensitivity analyses excluding patients with ALS in which we found a consistent signal for survival benefit in those who decreased  to < 50 mm Hg. With regard to the subgroup analysis in the subjects with ALS, although there was a trend toward improved survival, we are heavily limited in our ability to detect statistically significant differences due to the small sample size of this subgroup.
to < 50 mm Hg. With regard to the subgroup analysis in the subjects with ALS, although there was a trend toward improved survival, we are heavily limited in our ability to detect statistically significant differences due to the small sample size of this subgroup.
Both in uni- and multivariable analyses, the baseline 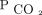 was not significantly associated with higher mortality. This could reflect that
was not significantly associated with higher mortality. This could reflect that 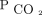 correction mitigates the mortality risk attributable to baseline hypercapnia. The fact that, during the follow-up period, we were able to achieve a mean
correction mitigates the mortality risk attributable to baseline hypercapnia. The fact that, during the follow-up period, we were able to achieve a mean 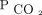 , of 50 mm Hg, for all baseline
, of 50 mm Hg, for all baseline  categories, (including the most extreme cases of
categories, (including the most extreme cases of 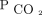 > 70 mm Hg) could account for the neutral effect of baseline hypercapnia in mortality and supports the notion that hypercapnia is a modifiable risk factor rather than just a marker of severity. In addition, a change of
> 70 mm Hg) could account for the neutral effect of baseline hypercapnia in mortality and supports the notion that hypercapnia is a modifiable risk factor rather than just a marker of severity. In addition, a change of  may be more indicative of the trajectory of the disease and reflects outcomes more strongly than a snapshot in time, such as a baseline
may be more indicative of the trajectory of the disease and reflects outcomes more strongly than a snapshot in time, such as a baseline 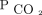 .
.
Although there is increasing evidence of the benefits of NIV on quality of life and symptom burden,35 little is known about how specific NIV management strategies impact survival. NIV for the management of chronic respiratory failure has focused primarily on hours of usage per day as opposed to specific physiologic variables. Adherence goals have been derived largely from insurance coverage criteria for the use of CPAP therapy for sleep apnea,36 and studies have shown a mortality benefit in patients using NIV for > 4 h/d.12,37 However, 4 h may grossly underestimate the hours of use needed to achieve normocapnia. Patients' ventilatory needs may vary from a few hours per days to 24 h/d depending on the severity of the disease and underlying pathophysiology. Therefore, although the hours of use may be sufficient for CPAP therapy secondary to obstructive sleep apnea, our study bolsters the argument for a shift in the way we manage NIV in chronic respiratory failure toward prioritizing 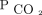 over hourly usage alone.
over hourly usage alone.
It is unknown whether targeting an absolute  threshold (eg, <50 mm Hg) or a
threshold (eg, <50 mm Hg) or a  percent reduction has superior outcomes. Given the equipoise, we feel this creates a clinical research question that is ripe for a future randomized control trial that compares CO2 reduction strategies in patients with chronic hypercapnia. Until further evidence is available, the ideal strategy may depend on the degree of baseline
percent reduction has superior outcomes. Given the equipoise, we feel this creates a clinical research question that is ripe for a future randomized control trial that compares CO2 reduction strategies in patients with chronic hypercapnia. Until further evidence is available, the ideal strategy may depend on the degree of baseline 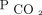 elevation, comorbidities, patient comfort with NIV settings, and aligning treatment strategy with patient goals of care.
elevation, comorbidities, patient comfort with NIV settings, and aligning treatment strategy with patient goals of care.
Arterial blood gas testing remains the standard assessment for 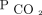 . However, there are many barriers to its regular use in the out-patient setting, including access to a blood gas analyzer and patient discomfort. PtcCO2 monitors provide an alternative, noninvasive method for longitudinal monitoring of alveolar ventilation. PtcCO2 has been shown to correlate with
. However, there are many barriers to its regular use in the out-patient setting, including access to a blood gas analyzer and patient discomfort. PtcCO2 monitors provide an alternative, noninvasive method for longitudinal monitoring of alveolar ventilation. PtcCO2 has been shown to correlate with  , with limits of agreement of –6 to 6 mm Hg when using modern sensors placed on the earlobe at a temperature of 42°C.38 This technique is practical for trending
, with limits of agreement of –6 to 6 mm Hg when using modern sensors placed on the earlobe at a temperature of 42°C.38 This technique is practical for trending 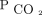 during titration of NIV. In addition, PtcCO2 has been used to diagnose respiratory failure in NMDs and to aid in the decision making of in-patient NIV initiation in a small case series.39 The high cost likely hinders the dissemination of PtcCO2 monitors. End-tidal pressure of expired CO2 also provides noninvasive continuous monitoring of
during titration of NIV. In addition, PtcCO2 has been used to diagnose respiratory failure in NMDs and to aid in the decision making of in-patient NIV initiation in a small case series.39 The high cost likely hinders the dissemination of PtcCO2 monitors. End-tidal pressure of expired CO2 also provides noninvasive continuous monitoring of 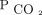 levels amenable to an out-patient setting. End-tidal pressure of expired CO2 is less costly than PtcCO2, which makes it a potentially attractive noninvasive option.
levels amenable to an out-patient setting. End-tidal pressure of expired CO2 is less costly than PtcCO2, which makes it a potentially attractive noninvasive option.
However, the accuracy of end-tidal pressure of expired CO2 is limited by inherent differences in 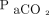 and end-tidal pressure of expired CO2 due to dead space, the effect of increasing age and ventilation/perfusion mismatch, and of decreasing with large tidal volume.40 Therefore, end-tidal pressure of expired CO2 may be error prone in patients who are spontaneously breathing for 2 reasons: (1) patients with chronic respiratory failure have frequent ventilation/perfusion mismatches, and (2) patients who require continuous NIV will experience mask leakage with measurements.41 Finally, serum bicarbonate has been used as a surrogate for blood
and end-tidal pressure of expired CO2 due to dead space, the effect of increasing age and ventilation/perfusion mismatch, and of decreasing with large tidal volume.40 Therefore, end-tidal pressure of expired CO2 may be error prone in patients who are spontaneously breathing for 2 reasons: (1) patients with chronic respiratory failure have frequent ventilation/perfusion mismatches, and (2) patients who require continuous NIV will experience mask leakage with measurements.41 Finally, serum bicarbonate has been used as a surrogate for blood  levels and may give a general sense of responses to NIV. However, primary metabolic alkalosis, including the use of diuretics, may limit its practical use for the monitoring of alveolar ventilation.
levels and may give a general sense of responses to NIV. However, primary metabolic alkalosis, including the use of diuretics, may limit its practical use for the monitoring of alveolar ventilation.
We were unable to reduce 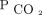 at 6–12 months in 12% of our subjects who were hypercapnic. Some subjects did not use NIV regularly or for sufficient hours per day to reduce diurnal
at 6–12 months in 12% of our subjects who were hypercapnic. Some subjects did not use NIV regularly or for sufficient hours per day to reduce diurnal 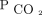 . Other subjects had such severe thoracic or lung disease that we were unable to achieve settings that effectively reversed hypercapnia. Such difficulties highlight the complexities involved in personalizing the management of chronic respiratory failure to each patient. PtcCO2 should be used in conjunction with a full understanding of device downloads to optimize home mechanical ventilation strategies. An inability to reduce
. Other subjects had such severe thoracic or lung disease that we were unable to achieve settings that effectively reversed hypercapnia. Such difficulties highlight the complexities involved in personalizing the management of chronic respiratory failure to each patient. PtcCO2 should be used in conjunction with a full understanding of device downloads to optimize home mechanical ventilation strategies. An inability to reduce 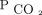 despite adjustments to ventilator settings and increasing hourly usage should prompt discussing advanced goals of care planning, including tracheostomy and/or palliative care for symptom management and end-of-life care planning.
despite adjustments to ventilator settings and increasing hourly usage should prompt discussing advanced goals of care planning, including tracheostomy and/or palliative care for symptom management and end-of-life care planning.
We acknowledge several limitations of our study. Due to the study's retrospective design, we cannot attribute causality or exclude the role of unmeasured confounders in the outcome. The subjects in our single-center study were followed up in a specialized clinic with expertise in home mechanical ventilation and PtcCO2 monitoring. Therefore, our findings are prone to selection bias and may not be generalizable to settings with fewer resources. The majority of 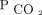 measurements were taken by using a PtcCO2 monitor, which is prone to bias compared with the accepted standard arterial blood gases. In addition, the placement of the transcutaneous sensor on the forehead has not been widely studied40 and could have introduced measurement bias.
measurements were taken by using a PtcCO2 monitor, which is prone to bias compared with the accepted standard arterial blood gases. In addition, the placement of the transcutaneous sensor on the forehead has not been widely studied40 and could have introduced measurement bias.
Conclusions
 reductions were associated with improved survival in chronic hypercapnic respiratory failure, a condition associated with high mortality. Given the strong association between improved
reductions were associated with improved survival in chronic hypercapnic respiratory failure, a condition associated with high mortality. Given the strong association between improved 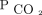 and survival, our study sets the rationale for larger prospective studies that explore this association and its potential role in improving clinically meaningful outcomes. In addition, our study highlights the importance of a home mechanical ventilation program with integrated PtcCO2 monitoring. Future work should focus on PtcCO2 technology dissemination to a wider clinical audience to facilitate multi-center cohort studies on how PtcCO2 reduction affects clinical outcomes, including hospitalizations and readmissions.
and survival, our study sets the rationale for larger prospective studies that explore this association and its potential role in improving clinically meaningful outcomes. In addition, our study highlights the importance of a home mechanical ventilation program with integrated PtcCO2 monitoring. Future work should focus on PtcCO2 technology dissemination to a wider clinical audience to facilitate multi-center cohort studies on how PtcCO2 reduction affects clinical outcomes, including hospitalizations and readmissions.
Footnotes
- Correspondence: Philip J Choi MD, Division of Pulmonary and Critical Care Medicine - University of Michigan, 3916 Taubman Center, 1500 E. Medical Center Dr, Ann Arbor, MI 48109. E-mail: pchoi{at}med.umich.edu
Dr Ackrivo discloses relationships with Hillrom and the National Institutes of Health National Heart, Lung and Blood Institute. Dr Labaki discloses relationships with Konica Minolta and Continuing Education Alliance. Dr Hansen-Flaschen discloses relationships with Revalesio Corporation. Dr Hyzy discloses relationships with Merck, Boehringer Ingelheim, Cour Pharmaceuticals, NOTA-Laboratories, Springer Website, UpToDate, CHEST Foundation and the NHLBI PETAL Network. Dr Choi discloses relationships with Breas Medical US. Dr Hsu discloses relationships with the National Kidney Foundation and PLOS ONE. The rest of the authors have no conflicts of interest.
Supplementary material related to this paper is available at http://www.rcjournal.com.
See the Related Editorial on Page 1775
- Copyright © 2023 by Daedalus Enterprises