Abstract
BACKGROUND: Bronchodilator delivery via a high-flow nasal cannula (HFNC) has generated interest in recent years. The efficacy of in-line vibrating mesh nebulizers with an HFNC during COPD exacerbation is limited. The aim of this study was to evaluate the clinical response of subjects with COPD exacerbation who require bronchodilator therapy (anticholinergic and β-agonist) by using a vibrating mesh nebulizer in line with an HFNC.
METHODS: This was a prospective single-center study performed in a respiratory intermediate care unit that enrolled patients with a diagnosis of COPD exacerbation who required noninvasive ventilation on admission. All the subjects underwent noninvasive ventilation breaks with an HFNC. After clinical stability, pulmonary function tests were performed to assess changes in FEV1 and clinical parameters before and after bronchodilation by using a vibrating mesh nebulizer in line with an HFNC.
RESULTS: Forty-six patients with COPD exacerbation were admitted. Five patients who did not use noninvasive ventilation and 10 patients who did not receive bronchodilator treatment with a vibrating mesh nebulizer were excluded. Thirty-one were selected, but 1 subject was secondarily excluded due to loss of data. Finally, 30 subjects were included. The primary outcome was spirometric changes in FEV1. The mean ± SD FEV1 before receiving bronchodilator treatment by using a vibrating mesh nebulizer in line with an HFNC was 0.74 ± 0.10 L, and, after receiving treatment, the mean ± SD FEV1 changed to 0.88 ± 0.12 L (P < .001). Similarly, the mean ± SD FVC increased from 1.75 ± 0.54 L to 2.13 ± 0.63 L (P < .001). Considerable differences were observed in breathing frequency and heart rate after receiving bronchodilator treatment. No relevant changes were observed in the Borg scale or SpO2 after treatment. The mean clinical stability recorded was 4 d.
CONCLUSIONS: In subjects with COPD exacerbation, bronchodilator treatment by using a vibrating mesh nebulizer in line with an HFNC showed a mild but significant improvement in FEV1 and FVC. In addition, a decrease in breathing frequency was observed, suggesting a reduction in dynamic hyperinflation.
Introduction
One in 10 adults in the world's population has COPD, which causes some 3.2 million deaths a year and has become 1 of the 3 most common causes of death worldwide.1,2 The main burden of COPD mortality is seen in low- and middle-income countries.3 Bronchodilator therapy is currently the main pharmacological treatment, and noninvasive ventilation (NIV) is an effective and evidence-based therapeutic tool in patients with COPD exacerbation.4,5 High-flow nasal cannula (HFNC) has gained popularity in recent years and has been proposed as an alternative in patients with COPD exacerbation for breaks in or intolerance to NIV.6 In subjects with COPD exacerbation, HFNC has been shown to reduce 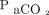 levels,7,8 breathing frequency, and decrease work of breathing, similar to NIV.9
levels,7,8 breathing frequency, and decrease work of breathing, similar to NIV.9
An HFNC delivers a heated and humidified air–oxygen mixture to the patient, with 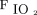 that ranges from 0.21 to 1.0 and a flow up to 60 L/min through a large-bore nasal cannula.10 The use of an in-line vibrating mesh nebulizer during HFNC therapy is a relatively novel combination; vibrating mesh nebulizers do not alter the flow or
that ranges from 0.21 to 1.0 and a flow up to 60 L/min through a large-bore nasal cannula.10 The use of an in-line vibrating mesh nebulizer during HFNC therapy is a relatively novel combination; vibrating mesh nebulizers do not alter the flow or  delivered by an HFNC because no oxygen source is required for operation.11 Clinical studies in subjects with stable COPD have demonstrated a satisfactory bronchodilator response by an HFNC with no significant differences compared with a jet nebulizer.12,13 Using noninvasive pulmonary function tests (PFT), the aim of this study was to evaluate the clinical response of subjects with COPD exacerbation who received bronchodilator therapy (anticholinergic and β-agonist) via a vibrating mesh nebulizer in line with an HFNC.
delivered by an HFNC because no oxygen source is required for operation.11 Clinical studies in subjects with stable COPD have demonstrated a satisfactory bronchodilator response by an HFNC with no significant differences compared with a jet nebulizer.12,13 Using noninvasive pulmonary function tests (PFT), the aim of this study was to evaluate the clinical response of subjects with COPD exacerbation who received bronchodilator therapy (anticholinergic and β-agonist) via a vibrating mesh nebulizer in line with an HFNC.
Quick Look
Current Knowledge
High-flow nasal cannula has gained increased use in patients with COPD exacerbation due to its well-described physiologic and clinical effects, in addition to being a comfortable and easy-to-use interface. This device can be an alternative to noninvasive ventilation in case of intolerance or as an alternative during noninvasive ventilation breaks. The use of bronchodilators is a mainstay in the treatment of COPD.
What this article adds to our knowledge
In severe COPD exacerbation we demonstrated a positive response to bronchodilator therapy with vibrating mesh nebulizers in line with high-flow nasal cannula. This bronchodilator effect was related to a substantial improvement in the subjects' pulmonary function and clinical variables. Therefore, the application of bronchodilators in line with vibrating mesh nebulizers and high-flow nasal cannula is possible without interrupting respiratory treatment, and no adverse events were observed.
Methods
Study Design
This was a prospective single-center study. Institutional review board reviewed the protocol and authorized prospective data collection (code register 2263). Informed written consent was obtained from all the subjects before inclusion in the study.
Subjects
Patients with a previous diagnosis of COPD who were admitted to the respiratory intermediate care unit within the Hospital de Agudos Juan A. Fernández with COPD exacerbation and required NIV for acute hypercapnic respiratory failure (pH ≤ 7.35, with a 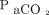 ≥ 45 mm Hg)5 were selected for the study. Underlying COPD could be documented by spirometry and defined by an FEV1/FVC < 0.7014 or, alternatively, highly suspected underlying COPD. Subjects with suspected underlying COPD without previous spirometry should have a history of smoking and emphysema on chest radiograph or computed tomography scan without other reasons for respiratory acidosis.
≥ 45 mm Hg)5 were selected for the study. Underlying COPD could be documented by spirometry and defined by an FEV1/FVC < 0.7014 or, alternatively, highly suspected underlying COPD. Subjects with suspected underlying COPD without previous spirometry should have a history of smoking and emphysema on chest radiograph or computed tomography scan without other reasons for respiratory acidosis.
Exclusion criteria were the following: inability to cooperate, inability to perform PFTs, unstable hemodynamics (systolic blood pressure < 90 mm Hg, atrial fibrillation), a history of asthma, cystic fibrosis, morbid obesity (body mass index > 40 kg/m2) thoracic deformities, previous known hypersensitivity to salbutamol, or pregnancy. All subjects in this study received bronchodilators via a vibrating mesh nebulizer in line with NIV from admission until clinical stabilization, NIV breaks were performed with an HFNC. Measurements were performed once subjects met the stabilty criteria. Frequency < 35 breaths/min, Glasgow coma scale score of 15, the need for intermittent NIV < 6 h, and the need for ≤ 4 bronchodilators per day.
Interventions
After a ≥ 6-h washout period without bronchodilator nebulization, the subjects were treated with bronchodilator therapy by using a vibrating mesh nebulizer in line with an HFNC.
HFNC
HFNC therapy was administered via Airvo2 (Fisher & Paykel, Auckland, New Zealand) through nasal prongs by using a medium-sized cannula, with a gas flow of 30 L/min, which allowed 100% relative humidity at 34°C, and 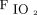 to maintain
to maintain 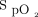 of 88%–92%.
of 88%–92%.
Nebulization
Nebulizer placement was as follows: according to the manufacturer's recommendations, the nebulizer was placed in the outlet of the humidifier for Airvo2 (with Airvo2 nebulizer adapter designed specifically for the Aerogen Solo). Medication was salbutamol (2.5 mg) and ipratropium bromide (0.5 mg) were provided through the vibrating mesh nebulizer (Aerogen Solo nebulizer and Aerogen Pro-X controller, Aerogen Galway, Ireland). The session was set at 30 min, and the complete delivery of bronchodilators was confirmed.
Data Collection
Demographic data were collected on admission to the respiratory intermediate care unit in conjunction with clinical parameters and laboratory blood test. Clinical parameters were measured before performing PFTs; dyspnea was assessed by using the Borg scale, which ranges from 0 to 10 points, with a higher score indicating maximum dyspnea. All PFTs were performed by using a spirometer (Spirolab III, MIR, Rome, Italy) before bronchodilator therapy and 60 min after bronchodilator therapy through the vibrating mesh nebulizer in line with an HFNC. For the performance of the PFTs, the HFNC was removed; for each test, 2 measurements of FEV1 and FVC were performed, and the best of them was recorded. The spirometry procedure was performed by following the American Thoracic Society/European Respiratory Society guidelines14 for standardization of PFT.
Outcomes
The primary outcome was change in FEV1 after bronchodilator therapy via a vibrating mesh nebulizer in line with an HFNC. Secondary outcomes included FVC changes and clinical parameters (breathing frequency, heart rate, 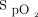 ) and dyspnea (Borg scale).
) and dyspnea (Borg scale).
Statistical Analysis
Continuous variables are presented as mean and SD (if data were normally distributed) and median and interquartile range (IQR) values (if data were not normally distributed). Categorical variables were described as frequency rates and percentages. Means for continuous variables were compared by paired t tests or analysis of variance test. Proportions of categorical variables were compared by using the chi-square test or Fisher exact test. P < .05 was considered statistically significant. The statistical analysis was performed by using R Studio (Version 1.3.1093, R Foundation for Statistical Computing, Vienna, Austria).
Results
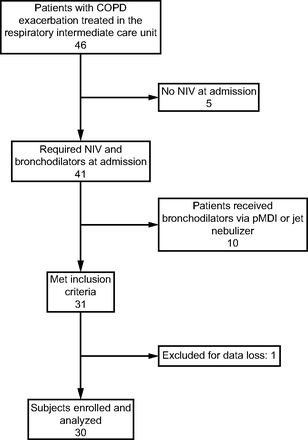
Forty-six patients with COPD exacerbation were admitted. Five patients who did not use NIV at admission and 10 patients who did not receive bronchodilator treatment with a vibrating mesh nebulizer were excluded. Thirty one were selected, but one subject was secondarily excluded due to loss of data in the system. Finally, 30 subjects were included from September 2021 to July 2022 (Fig. 1). There were 23 subjects with severe COPD classification according to GOLD (Global Initiative for Chronic Obstructive Lung Disease) (Table 1).
Flow chart. NIV = noninvasive ventilation; pMDI = pressurized metered-dose inhaler.
Demographic and Baseline Characteristics of Subjects with COPD Exacerbation Admitted to Respiratory Intermediate Care Unit (N= 30)
The primary outcome was spirometric changes in FEV1. The mean ± SD FEV1 before receiving bronchodilator treatment when using a vibrating mesh nebulizer in line with an HFNC was 0.74 ± 0.10 L and after receiving treatment the mean ± SD FEV1 changed to 0.88 ± 0.12 L (P < .001) (Table 1). The FEV1 increased in 83% of the subjects (25 of the 30 subjects). Secondary outcome measures included FVC and clinical parameters. Similarly, mean ± SD FVC increased from 1.75 ± 0.54 L to 2.13 ± 0.63 L (P < .001). The FVC increased in 83% (25 of the 30 subjects). Significant differences were observed in breathing frequency and heart rate after receiving bronchodilator treatment through a vibrating mesh nebulizer in line with an HFNC (Table 1) (P < .001). No significant changes were observed in Borg scale and 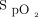 after treatment (Table 2). The mean ± SD clinical stability recorded was 4 ± 0.92 d. When PFTs were performed 60 min after aerosol therapy, the preset HFNC flow was restored and complete uninterrupted delivery of the dose by using the vibrating mesh nebulizer was noted for all aerosol therapy sessions, and no alarms were noted on the Airvo2 machine.
after treatment (Table 2). The mean ± SD clinical stability recorded was 4 ± 0.92 d. When PFTs were performed 60 min after aerosol therapy, the preset HFNC flow was restored and complete uninterrupted delivery of the dose by using the vibrating mesh nebulizer was noted for all aerosol therapy sessions, and no alarms were noted on the Airvo2 machine.
Changes in Pulmonary Function Tests and Clinical Parameters Before and After Bronchodilator Therapy
Discussion
In this single-center study, the subjects with COPD exacerbation showed improvement in FEV1 and FVC after receiving bronchodilator therapy by using a vibrating mesh nebulizer in line with an HFNC, which suggests a positive bronchodilator effect. Physiologic effects of HFNC are well described in the literature; the application of an HFNC can facilitate the elimination of CO2 by elevated gas flows.10,15 This promotes the flushing of anatomic dead space of the upper airway, and the CPAP effect could contribute to decrease the work of breathing caused by expiratory air flow obstruction by compensating for intrinsic PEEP.16,17 A recent study was able to confirm these physiologic effects by proving a reduction in inspiratory effort and neuroventilatory drive in stable and COPD exacerbation subjects.16,18,19
For these reasons, we consider it an attractive combination to perform aerosol therapy through a vibrating mesh nebulizer in line with an HFNC. A common practice includes positioning a nebulizer face mask over the nasal cannula during therapy. This setup considerably reduces the amount of aerosol being inhaled by the patient and, in some cases, reduces it to as low as ∼1% of the nominal dose placed in the nebulizer for adults, and lower still in newborn and pediatric patients, with levels reported to be between 0.1% and 0.93% of the nominal dose.17,20 The optimal configuration for nebulization through the HFNC system has been shown to be placement dry side of the humidifier and with gas flow as low as possible but at a level that can be tolerated by the patient.21 Previous studies administered aerosol to subjects at a gas flow that did not exceed 30 L/min.12,22 However, we decreased the gas flow to 30 L/min to facilitate optimal concurrent bronchodilator therapy. All the subjects tolerated the decrease in flow without adverse events. Further, this is in line with international clinical practice, in which it is reported that, during 30% of aerosol therapy sessions, HFNC gas flow is reduced.23
FEV1 and FVC are both known to be reliable parameters for measurement of expiratory air flow obstruction and volume retention, and have been demonstrated to be easily reproducible in a large proportion of subjects when obtained by trained specialists.24 In our study, the usual criteria for reversibility (ie, 12% increase and 200 mL) were not reached. Our data are similar to those reported by Beuvon et al,25 in which they performed bronchodilation with salbutamol via a vibrating mesh nebulizer in line with an HFNC, FEV1 showed changes of 9.5% in their study population. Our study showed 19% changes in FEV1 in a population with mostly severe (GOLD IV) COPD. Reminiac et al12 showed a > 16% increase in FEV1 when using a vibrating mesh nebulizer in line with an HFNC in subjects with stable asthma and COPD.
A recent study indicates that the prevalence of bronchodilator reversibility in subjects with COPD was only 17% when these usual criteria were met.26 However, a 5%–10% change in FEV1 from baseline values is considered clinically relevant, whereas a change of <3% has been considered not to be clinically relevant.27 Therefore, a slight increase in FEV1 may result in a reduction in residual volume and delay in the onset of dynamic hyperinflation during tachypnea.28,29 Of note, those 3 studies also made use of an HFNC system with a vibrating mesh nebulizer, and the temperature, flow, and cannula size used were the same as that described herein.12,22,25 We reported increased FVC after bronchodilator nebulization, which could be considered a consequence of a reduction in lung hyperinflation.30,31 In fact, there is a certain group of patients in whom bronchodilation can induce changes in FVC rather than FEV1. This has been associated with the effect of airway inflation due to loss of elastic recoil or to spatial competition.31 The first limitation of our study was the small number of subjects and, second, only 2 spirometric measurements were performed to avoid subject fatigue.
Conclusions
In subjects with COPD exacerbation, bronchodilator treatment by using a vibrating mesh nebulizer in line with an HFNC showed a mild but substantial improvement in FEV1 and FVC. In addition, a decrease in breathing frequency was observed, which suggests a reduction in dynamic hyperinflation.
Footnotes
- Correspondence: Nicolas Colaianni-Alfonso, Respiratory Intermediate Care Unit, Hospital Juan A. Fernández, Av. Cerviño 3356, C1425 Ciudad Autónoma Buenos Aires, Argentina. E-mail: nicolkf{at}gmail.com
See the Related Editorial on Page 856
The study location was Hospital Juan A. Fernández, Respiratory Intermediate Care Unit, Ciudad Autónoma Buenos Aires, Argentina.
No funding was received to assist with preparation of this manuscript.
Dr MacLoughlin is an employee of Aerogen Limited. The other authors have disclosed no conflicts of interest.
- Copyright © 2023 by Daedalus Enterprises