- electrical impedance tomography
- acute respiratory distress syndrome
- COVID-19
- phenotype
- precision medicine
- morphofunctional classification.
Introduction
Since the first description of ARDS, bilateral alveolar infiltrates on chest radiography have been recognized as characteristic of this syndrome, combined with hypoxemia and low respiratory system compliance ( ).1 Due to its simplicity, chest radiography is still a pillar of the actual ARDS definition.2 However, only the advance to chest computed tomography (CT) allowed clinicians to precisely identify the site of lung disease, revealing the heterogeneous distribution of lung injury in patients with ARDS.3,4 As with previous ARDS descriptions, CT studies reported a variety of areas with pulmonary involvement in subjects with COVID-19,5,6 such as multilobar ground-glass opacities and consolidation located mainly in the gravity-dependent regions.
).1 Due to its simplicity, chest radiography is still a pillar of the actual ARDS definition.2 However, only the advance to chest computed tomography (CT) allowed clinicians to precisely identify the site of lung disease, revealing the heterogeneous distribution of lung injury in patients with ARDS.3,4 As with previous ARDS descriptions, CT studies reported a variety of areas with pulmonary involvement in subjects with COVID-19,5,6 such as multilobar ground-glass opacities and consolidation located mainly in the gravity-dependent regions.
The use of a chest CT to phenotype ARDS as a focal or non-focal disease is also advocated to allow personalized mechanical ventilation.7 However, the use of CT is limited by the ionizing radiation and the need for patient transfer.8 In this scenario, electrical impedance tomography (EIT) has been used at the bedside to monitor pulmonary function in real time during mechanical ventilation.9,10 In this report, we aimed to describe the distribution pattern of hypoventilated regions (ie, areas with deteriorated lung function) in patients with COVID-19–related ARDS under invasive mechanical ventilation with the use of EIT. In addition, we assessed the relationship between the distribution pattern of hypoventilated regions with CRS and gas exchange (PaO2/FIO2).
Methods
This study is a secondary analysis of a clinical trial (https://clinicaltrials.gov, NCT 05024500) that consecutively monitored subjects with EIT who had been admitted to a tertiary ICU from October 2020 to June 2021. All the subjects were ventilated in the volume-controlled mode (tidal volume of 6 mL/kg of predicted body weight) for < 72 h, with PEEP of 10 cm H2O as recommended previously by Villar et al.11 In this analysis, we excluded subjects on assisted or spontaneous ventilation modes and with a PaO2/FIO2 of 300 mm Hg on the day of assessment.
The EIT images were acquired by using Enlight-1800 (Timpel Medical, São Paulo, Brazil) with the electrode strap positioned around the patient's chest at the axillar level. The subjects were in the supine position, with the head tilted at 30°. The lung images were divided into 4 regions of interest (ROI): upper right (UR), lower right (LR), upper left (UL), and lower left (LL). The regional distribution of ventilation was determined as a percentage of the change in impedance for each quadrant in relation to the total change in impedance:
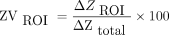 The distribution of hypoventilated regions was stratified into 4 patterns (or phenotypes): pattern 1, preserved bilateral dorsal ventilation (
The distribution of hypoventilated regions was stratified into 4 patterns (or phenotypes): pattern 1, preserved bilateral dorsal ventilation ( > 40%); pattern 2, bilateral dorsal hypoventilation (
> 40%); pattern 2, bilateral dorsal hypoventilation (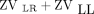 < 40%); pattern 3, unilateral dorsal hypoventilation (
< 40%); pattern 3, unilateral dorsal hypoventilation (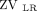 or
or 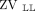 < 20%); and pattern 4, unilateral hypoventilation (
< 20%); and pattern 4, unilateral hypoventilation (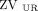 +
+ 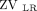 or
or 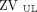 +
+ 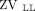 < 40%) (Fig. 1A).
< 40%) (Fig. 1A).
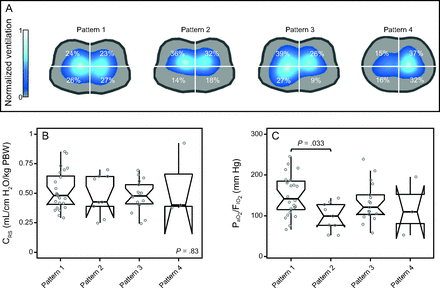
A: Illustration of the distribution of hypoventilated regions patterns detected by electrical impedance tomography (EIT): pattern 1, preserved bilateral dorsal ventilation ( > 40%); pattern 2, bilateral dorsal hypoventilation (
> 40%); pattern 2, bilateral dorsal hypoventilation ( < 40%); pattern 3, unilateral dorsal hypoventilation (
< 40%); pattern 3, unilateral dorsal hypoventilation ( < 20%); and pattern 4, unilateral hypoventilation (
< 20%); and pattern 4, unilateral hypoventilation ( or
or  < 40%). The color scale of the EIT image was normalized by the sum of the impedance values within the lung area (the lighter the blue, the greater the regional ventilation). B: Respiratory-system compliance (CRS) normalized by predicted body weight. C: PaO2/FIO2 according to the hypoventilation pattern. Box plots in panels B and C express median, 25% and 75% quartiles, minimum and maximum, and outliers (>1.5 interquartile length values).
< 40%). The color scale of the EIT image was normalized by the sum of the impedance values within the lung area (the lighter the blue, the greater the regional ventilation). B: Respiratory-system compliance (CRS) normalized by predicted body weight. C: PaO2/FIO2 according to the hypoventilation pattern. Box plots in panels B and C express median, 25% and 75% quartiles, minimum and maximum, and outliers (>1.5 interquartile length values).
Respiratory system mechanics and arterial blood gases were assessed during EIT assessment. 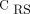 was calculated as expiratory tidal volume divided by the difference between plateau pressure and total PEEP. The plateau pressure and total PEEP were measured by using an end-inspiratory occlusion of 0.5 s and an end-expiratory occlusion of 2 s, respectively. CRS was normalized by the predicted body weight to account for differences in height and sex (eg, a normalized compliance of 0.5 mL/cm H2O/kg predicted body weight would correspond to 35 mL/cm H2O for a 70-kg patient).
was calculated as expiratory tidal volume divided by the difference between plateau pressure and total PEEP. The plateau pressure and total PEEP were measured by using an end-inspiratory occlusion of 0.5 s and an end-expiratory occlusion of 2 s, respectively. CRS was normalized by the predicted body weight to account for differences in height and sex (eg, a normalized compliance of 0.5 mL/cm H2O/kg predicted body weight would correspond to 35 mL/cm H2O for a 70-kg patient).
Statistical Analysis
Continuous variables were expressed as median (interquartile range), and categorical variables were expressed as frequencies (percentages). Differences between groups were assessed with Kruskal-Wallis test, followed by the Dunn post hoc test. Differences were considered significant with P < .05. The analysis was conducted by using RStudio (v1.1.456; PBC, Boston, Massachusetts) and R Statistical Software (v3.6.2; R Foundation for Statistical Computing, Vienna, Austria).
Results
A total of 53 subjects were included in this analysis (Table 1): 24 subjects (45%) showed preserved bilateral dorsal ventilation (pattern 1), 9 subjects (17%) showed bilateral dorsal hypoventilation (pattern 2), 17 subjects (32%) showed unilateral dorsal hypoventilation (pattern 3), and 3 subjects (5.7%) showed unilateral hypoventilation (pattern 4). 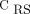 was comparable among the distribution pattern of hypoventilated regions (Fig. 1B). PaO2/FIO2 was lower in pattern 2 compared with pattern 1 (Fig. 1C).
was comparable among the distribution pattern of hypoventilated regions (Fig. 1B). PaO2/FIO2 was lower in pattern 2 compared with pattern 1 (Fig. 1C).
Characteristics of the Subjects at Baseline
Discussion
To our knowledge, this is the first study to demonstrate the utility of EIT in detecting the heterogeneous topographic distribution of lung disease in the subjects with COVID-19–related ARDS. Analysis of our data indicates a higher prevalence of subjects with preserved bilateral dorsal ventilation, followed by unilateral and bilateral dorsal hypoventilation. Analysis of these results suggests a heterogeneous EIT-topographic distribution of the lung disease in ARDS, as reported by previous CT studies.4,-,6
We defined the EIT patterns (or phenotypes) based on a previous CT study,7 which dichotomized the phenotype of ARDS as a non-focal (corresponding to EIT pattern 1) or focal disease (corresponding to EIT patterns 2, 3, and 4). EIT patterns 3 and 4 were idealized to allow the classification of unilateral lung involvement. We did not include in the current classification the hypoventilation alone in the anterior quadrants due to the risk of interference by the heart area and pulmonary hyperinflation, reducing the ventilation in non-gravity–dependent regions. The threshold of ventilation distribution was defined based on the known range of error between EIT and CT for anterior versus posterior and right versus left regions (bias of 0% and limits of agreement of 10%).12 Thus, for instance, the EIT image that shows dorsal ventilation < 40% has a high accuracy to suggest the existence of a regional lung impairment (eg, atelectasis, consolidation).
This report intends to show how EIT can add information to identifying topographic disease distribution in ARDS. For instance, analysis of our data suggests that the subjects with preserved bilateral dorsal ventilation (pattern 1) had comparable 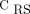 with those with bilateral dorsal hypoventilation (pattern 2, which is usually related to regional dorsal consolidations or atelectasis). Therefore, the identification of a patient with pattern 1 can exclude any hypothesis about focal disease presentation. At the same time, the combined information about EIT pattern 1 with high or low
with those with bilateral dorsal hypoventilation (pattern 2, which is usually related to regional dorsal consolidations or atelectasis). Therefore, the identification of a patient with pattern 1 can exclude any hypothesis about focal disease presentation. At the same time, the combined information about EIT pattern 1 with high or low 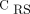 can address the severity and/or progression of a non-focal ARDS (eg, patients with minimal or severe diffuse ground-glass opacities).9 Furthermore, the global
can address the severity and/or progression of a non-focal ARDS (eg, patients with minimal or severe diffuse ground-glass opacities).9 Furthermore, the global 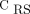 was not an informative variable to detect the extension of hypoventilated areas (eg, pattern 2 vs pattern 3 and pattern 2 vs pattern 4), and PaO2/FIO2 tended to be lower in the subjects with bilateral dorsal hypoventilation, possibly due to the increased shunt area.
was not an informative variable to detect the extension of hypoventilated areas (eg, pattern 2 vs pattern 3 and pattern 2 vs pattern 4), and PaO2/FIO2 tended to be lower in the subjects with bilateral dorsal hypoventilation, possibly due to the increased shunt area.
We believe that EIT bedside information supports a step forward in clinical studies, in testing ventilatory strategies tailored to lung imaging. For instance, body positioning, such as prone positioning, has frequently been used in patients with ARDS to improve oxygenation by promoting dorsal lung recruitment.13,14 We found that only one-sixth of our subjects had bilateral dorsal hypoventilation. On the other hand, the greater prevalence of unilateral dorsal hypoventilation may indicate the use of lateral positioning, with the sick lung region positioned up to favor alveolar recruitment.15 The small sample size from a single center limited a strong conclusion from our data. Thus, future studies are needed to investigate the role of EIT phenotypes on clinical outcomes. In conclusion, the present study demonstrates a heterogeneous EIT topographic distribution of lung disease in subjects with COVID-19–related ARDS. In addition, standard global parameters such as 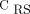 and
and 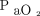 /
/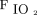 were almost indistinguishable among the EIT phenotypes.
were almost indistinguishable among the EIT phenotypes.
Footnotes
- Correspondence: Daniella C Brandão PhD, Physiotherapy Department, Universidade Federal de Pernambuco, Av. Jorn. Aníbal Fernandes, 173, Recife, Pernambuco 50740-560, Brazil. E-mail: daniella.brandao{at}ufpe.br
Ms Caetano and Dr Morais contributed equally to this work.
This work has been supported by the following Brazilian research agencies: Federal University of Pernambuco (UFPE- PROPG), Coordination for the Improvement of Higher Education Personnel (CAPES)-financial code 001, National Council for Scientific and Technological Development- (CNPq/ Ministry of Science, Technology, Innovation and Communications/Ministry of Health of Brazil) (403341/2020-5), and Foundation for the Support of Science and Technology of the State of Pernambuco- FACEPE (APQ-0249-4.08/20).
ClinicalTrials.gov registration NCT 05024500.
- Copyright © 2023 by Daedalus Enterprises