Introduction
Oxygenation targets are defined by maximum and minimum 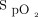 boundaries in an effort to avoid both hypoxemia and hyperoxemia. However, these limits are difficult to impose clinically, in part, due to confounding factors of
boundaries in an effort to avoid both hypoxemia and hyperoxemia. However, these limits are difficult to impose clinically, in part, due to confounding factors of 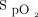 measurement accuracy.1 The impact of skin pigmentation on oximeter accuracy has been the subject of justified awareness in the scientific literature and lay press owing to potential exacerbation of health inequities.2 Other factors may have an impact at least as important for oxygen therapy management, including the choice of the
measurement accuracy.1 The impact of skin pigmentation on oximeter accuracy has been the subject of justified awareness in the scientific literature and lay press owing to potential exacerbation of health inequities.2 Other factors may have an impact at least as important for oxygen therapy management, including the choice of the 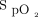 target3–6 and oximeter brand.7
target3–6 and oximeter brand.7
Several 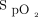 targets have been recommended for the management of patients with hypoxemic respiratory failure, from 92 ± 2%3 to 96 ± 2%.4,5 In patients who are spontaneously breathing and on oxygen therapy, the choice of the
targets have been recommended for the management of patients with hypoxemic respiratory failure, from 92 ± 2%3 to 96 ± 2%.4,5 In patients who are spontaneously breathing and on oxygen therapy, the choice of the 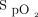 target has been shown to modulate oxygen flow, with an increase by >3-fold for 4% differences in
target has been shown to modulate oxygen flow, with an increase by >3-fold for 4% differences in 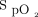 targets.8 This 3-fold increase in oxygen delivery may have a real impact in evaluating the patient's severity of illness, on the decision to escalate or de-escalate respiratory support, and for the oxygen utilization.9
targets.8 This 3-fold increase in oxygen delivery may have a real impact in evaluating the patient's severity of illness, on the decision to escalate or de-escalate respiratory support, and for the oxygen utilization.9
In addition, the oximeter brand may influence the oxygen flow required to maintain a target level of oxygenation.1 Indeed, it has recently been shown that the oximeter brand also influences 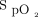 measurements, with a mean bias up to 4% among commonly used pulse oximeters.7 It is not known if the error related to the brand of the oximeter can have an additional impact on the choice of the
measurements, with a mean bias up to 4% among commonly used pulse oximeters.7 It is not known if the error related to the brand of the oximeter can have an additional impact on the choice of the 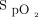 target and of what magnitude. The objective of this short-term physiologic study was to evaluate the impact of the combination of different
target and of what magnitude. The objective of this short-term physiologic study was to evaluate the impact of the combination of different 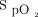 targets and oximeter brands on oxygen flow requirements and oxygenation parameters.
targets and oximeter brands on oxygen flow requirements and oxygenation parameters.
Methods
We conducted a prospective randomized crossover study in 20 ICU subjects who were stable and who required oxygen therapy delivered through a nasal canula after cardiac surgery (ClinicalTrials.gov registration NCT05590130). Subjects were prospectively included from December 2022 to March 2023 at our institution (Institut Universitaire de Cardiologie et de Pneumologie de Québec). Subjects without an adequate 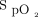 signal were excluded. The study was approved by the institutional ethics committee, and all the subjects provided signed informed consent. Four randomized periods of study in 10-min blocks were conducted, with a combination of 2 different
signal were excluded. The study was approved by the institutional ethics committee, and all the subjects provided signed informed consent. Four randomized periods of study in 10-min blocks were conducted, with a combination of 2 different 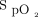 targets (90% and 94%) while using 2 different oximeters (Nonin, Plymouth, Minnesota; and Philips FAST, Eindhoven, Netherlands). The mean bias between these oximeters was 4% in our previous work.7 At the end of each period, we recorded the oxygen flow and obtained arterial blood gases. Arterial oxygen saturation,
targets (90% and 94%) while using 2 different oximeters (Nonin, Plymouth, Minnesota; and Philips FAST, Eindhoven, Netherlands). The mean bias between these oximeters was 4% in our previous work.7 At the end of each period, we recorded the oxygen flow and obtained arterial blood gases. Arterial oxygen saturation, 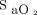 , was determined by multiwavelength oximetry (Radiometer ABL 800Flex OSM-3, Mississauga, Ontario, Canada). We compared the 4 periods for the oxygen flow (primary end point), the rate of occult hypoxemia (defined as
, was determined by multiwavelength oximetry (Radiometer ABL 800Flex OSM-3, Mississauga, Ontario, Canada). We compared the 4 periods for the oxygen flow (primary end point), the rate of occult hypoxemia (defined as 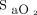 < 90% and
< 90% and 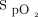 ≥ 90%) and occult hyperoxemia (defined as
≥ 90%) and occult hyperoxemia (defined as 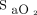 > 96% and
> 96% and 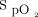 ≤ 96%), oxygen partial weaning (flow < 0.5 L/min) or complete weaning and the rate of high O2 flow requirements (>5 L/min) (secondary end points).
≤ 96%), oxygen partial weaning (flow < 0.5 L/min) or complete weaning and the rate of high O2 flow requirements (>5 L/min) (secondary end points).
Results
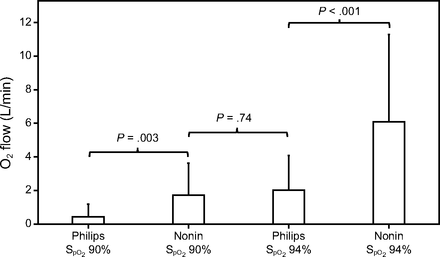
Twenty subjects were studied (mean ± SD age 68 ± 8 years), 16 were men (80%), all had light skin pigmentation (Fitzpatrick skin scale 1 or 2), which reflected the local population, none had shock. At baseline, 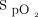 was mean ± SD 93.4 ± 1.8% and oxygen flow was 2.1 ± 1.4 L/min. Oxygen flow requirements in the different study periods are displayed in Figure 1. Differences in mean oxygen flow during monitoring with the Nonin with an
was mean ± SD 93.4 ± 1.8% and oxygen flow was 2.1 ± 1.4 L/min. Oxygen flow requirements in the different study periods are displayed in Figure 1. Differences in mean oxygen flow during monitoring with the Nonin with an 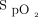 target of 90% and with the Philips with an
target of 90% and with the Philips with an 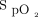 target of 94% were not statistically different (P = .74). However, all other comparisons for the oxygen flow requirements were statistically different. The influence of the oximeter brand on oxygen flow was of similar amplitude as the influence of
target of 94% were not statistically different (P = .74). However, all other comparisons for the oxygen flow requirements were statistically different. The influence of the oximeter brand on oxygen flow was of similar amplitude as the influence of 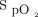 targets, as suggested by the oxygen ratio (Fig. 1). For the same
targets, as suggested by the oxygen ratio (Fig. 1). For the same 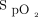 target, the oxygen flow was significantly increased by a factor 3 to 4 when using the Nonin oximeter in comparison with the Philips oximeter. With the same oximeter, the oxygen flow requirement was increased by a factor of 3.6 to 4.7 with the
target, the oxygen flow was significantly increased by a factor 3 to 4 when using the Nonin oximeter in comparison with the Philips oximeter. With the same oximeter, the oxygen flow requirement was increased by a factor of 3.6 to 4.7 with the 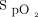 target of 94% versus 90%.
target of 94% versus 90%.
Mean oxygen flow utilization in the different study conditions that compared 2 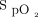 targets (90 and 94%) and 2 oximeters brand (Philips and Nonin): Philips 90, Nonin 90, Philips 94, and Nonin 94. The oxygen ratios were 1.2 (Philips 94/Nonin 90), 4.2 (Nonin 90/Philips 90), 3.1 (Nonin 94/Philips 94), 3.6 (Nonin 94/Nonin 90), 5.0 (Philips 94/Philips 90), and 15.3 (Nonin 94/Philips 90).
targets (90 and 94%) and 2 oximeters brand (Philips and Nonin): Philips 90, Nonin 90, Philips 94, and Nonin 94. The oxygen ratios were 1.2 (Philips 94/Nonin 90), 4.2 (Nonin 90/Philips 90), 3.1 (Nonin 94/Philips 94), 3.6 (Nonin 94/Nonin 90), 5.0 (Philips 94/Philips 90), and 15.3 (Nonin 94/Philips 90).
The combination of these factors resulted in greater discrepancies. Oxygen flow was reduced by a factor of 15 between the condition of a high 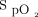 target attained with an oximeter that underestimated oxygenation (Nonin, 94%) and a low
target attained with an oximeter that underestimated oxygenation (Nonin, 94%) and a low 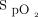 target attained with an oximeter that tended to overestimate oxygenation (Philips, 90%). This study does not consider the impact of skin pigment because all the subjects were light skinned. The data concerning the impact of the tested
target attained with an oximeter that tended to overestimate oxygenation (Philips, 90%). This study does not consider the impact of skin pigment because all the subjects were light skinned. The data concerning the impact of the tested 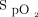 targets and oximeter brands as well as the combination of both on arterial blood gases and short-term clinical outcomes are displayed in Table 1. The rate of complete oxygen weaning was 55% in the Philips 90% period and 0 to 5% in other periods, P < .001. No subject had oxygen flow > 5 L/min during the Philips 90% period, whereas 8 subjects (40%) had high oxygen flows (mean ± SD of 10.9 ± 5.5 L/min) during the Nonin 94% period, P < .001. Oxygenation parameters (
targets and oximeter brands as well as the combination of both on arterial blood gases and short-term clinical outcomes are displayed in Table 1. The rate of complete oxygen weaning was 55% in the Philips 90% period and 0 to 5% in other periods, P < .001. No subject had oxygen flow > 5 L/min during the Philips 90% period, whereas 8 subjects (40%) had high oxygen flows (mean ± SD of 10.9 ± 5.5 L/min) during the Nonin 94% period, P < .001. Oxygenation parameters (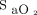 and
and 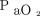 ) were similar during the Nonin 90% and Philips 94% periods. Conversely, there were statistically significant differences for the oxygenation parameters and for other comparisons, including a higher rate of occult hyperoxemia during the Nonin 94% period (Table 1).
) were similar during the Nonin 90% and Philips 94% periods. Conversely, there were statistically significant differences for the oxygenation parameters and for other comparisons, including a higher rate of occult hyperoxemia during the Nonin 94% period (Table 1).
Results of Oxygen Flow, Arterial Blood Gases, and Other Oxygenation and Outcome Parameters at the End of Each Study Period in the 20 Included Subjects
Discussion
In a population of subjects who required conventional oxygen therapy after cardiac surgery, the 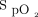 target, the oximeter brand and the two in combination had a major impact on oxygen utilization, oxygen weaning, and occult hyperoxemia. The same patient might require 15 times more oxygen, depending on the choice of the
target, the oximeter brand and the two in combination had a major impact on oxygen utilization, oxygen weaning, and occult hyperoxemia. The same patient might require 15 times more oxygen, depending on the choice of the 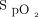 target and oximeter brand. The impact of a 4% difference for the
target and oximeter brand. The impact of a 4% difference for the 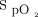 target and the oximeter brand had an equivalent impact on oxygen flow requirements. The
target and the oximeter brand had an equivalent impact on oxygen flow requirements. The 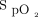 target and the oximeter brand combined had at least additive effects. More than half of the subjects were considered weaned from oxygen with one combination (Philips, 90%) whereas almost half required high oxygen flows with another combination (Nonin, 94%).
target and the oximeter brand combined had at least additive effects. More than half of the subjects were considered weaned from oxygen with one combination (Philips, 90%) whereas almost half required high oxygen flows with another combination (Nonin, 94%).
Although the impact of 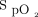 targets or oximeter brand has been overlooked, analysis of these data suggests that these simple parameters considered in isolation and more importantly in combination can have a relevant impact on day-to-day clinical management. These differences can alter important decisions related to hospital discharge, admission to intensive care, or escalation of respiratory support (conventional oxygen to nasal high flow to intubation) as well as for clinical research, particularly if oxygen-free days are used to describe patient outcomes.1,10 It should be noted that all the subjects in this study had light skin pigmentation and it is likely that in subjects with dark skin, the rate of oxygen weaning might be greater as well as the incidence of occult hypoxemia.11
targets or oximeter brand has been overlooked, analysis of these data suggests that these simple parameters considered in isolation and more importantly in combination can have a relevant impact on day-to-day clinical management. These differences can alter important decisions related to hospital discharge, admission to intensive care, or escalation of respiratory support (conventional oxygen to nasal high flow to intubation) as well as for clinical research, particularly if oxygen-free days are used to describe patient outcomes.1,10 It should be noted that all the subjects in this study had light skin pigmentation and it is likely that in subjects with dark skin, the rate of oxygen weaning might be greater as well as the incidence of occult hypoxemia.11
The results found in the present study are in line with previous reports. In the present study, the bias between the 2 tested oximeters was the same as in a previous study with a similar population (ICU subjects with light skin pigmentation who were stable).7 In addition, the impact on the oxygen flow requirements with a 4% difference in the 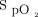 target was similar to what was found in a previous study.8 To our knowledge, no study previously reported the impact of the combination of these confounding factors on oxygen flow.
target was similar to what was found in a previous study.8 To our knowledge, no study previously reported the impact of the combination of these confounding factors on oxygen flow.
This study has some limitations. The study has a small sample size; however, the small number necessary to demonstrate an effect with the studied conditions, demonstrates that the effect is consistent for all the subjects. We included only subjects with light skin pigmentation, and the impact might have been different in other populations. It is likely that the occult hypoxemia would be more frequent in patients with dark skin pigmentation in the Philips 90% period. Finally, we only evaluated the short-term effects of the outcome and hospital stay may be related to other clinical and biologic determinants (eg, breathing frequency, fever, inflammation parameters in the cases of pneumonia).
This study shows that the choice of an 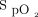 target, of the oximeter brand, and the combination of both have a clinically relevant impact, at least equivalent to the skin pigmentation factor. These data on oxygen use may also be relevant during a pandemic9 or in resource-constrained environments. In many low-income and lower-to-middle–income countries, access to oxygen remains a difficult priority to ensure adequate treatment for patients with acute respiratory failure.12,13
target, of the oximeter brand, and the combination of both have a clinically relevant impact, at least equivalent to the skin pigmentation factor. These data on oxygen use may also be relevant during a pandemic9 or in resource-constrained environments. In many low-income and lower-to-middle–income countries, access to oxygen remains a difficult priority to ensure adequate treatment for patients with acute respiratory failure.12,13
These confounders can also have an impact in the context of research, in which finding the optimal 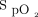 target seems to be a quest for the Holy Grail. The most recent randomized controlled studies that evaluated different
target seems to be a quest for the Holy Grail. The most recent randomized controlled studies that evaluated different 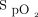 targets did not consider the confounding factors for
targets did not consider the confounding factors for 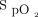 measurements.1,14 If the targets of 90%, 94%, or 98% are used undiscerningly, without consideration for oximeter brand or skin pigmentation, the impact on clinical management and on the results of clinical trials that compare different oxygenation targets may be conflicting.
measurements.1,14 If the targets of 90%, 94%, or 98% are used undiscerningly, without consideration for oximeter brand or skin pigmentation, the impact on clinical management and on the results of clinical trials that compare different oxygenation targets may be conflicting.
These parameters alone or in combination have a significant impact on oxygen management and must be taken into account when the 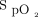 target is chosen for a given patient and for future research that seeks optimal oxygenation targets in patients with acute respiratory failure. When bias of individual oximeters are not considered, the difference in selected oxygen targets may result in similar
target is chosen for a given patient and for future research that seeks optimal oxygenation targets in patients with acute respiratory failure. When bias of individual oximeters are not considered, the difference in selected oxygen targets may result in similar 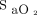 , defeating the objective of elucidating
, defeating the objective of elucidating 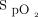 targets on outcomes in respiratory failure.
targets on outcomes in respiratory failure.
Although the world is rightly concerned over inaccuracies related to skin pigment (which demonstrates social awareness and may exacerbate health inequities), errors induced by the oximeter used are equally important (but often ignored) and, together with skin pigment, magnify errors. The simplicity of oximetry use belies a plethora of confounding factors that are frequently not considered and have a clinically important impact on patient management and outcomes in clinical trials.15
Acknowledgments
We thank Patricia Lizotte for her assistance in data collection and Serge Simard for statistical analysis.
Footnotes
- Correspondence: François Lellouche MD, Centre de Recherche de Institut Universitaire de Cardiologie et de Pneumologie de Québec, 2725, Chemin Sainte-Foy, G1V 4G5 Québec, QC, Canada. E-mail: francois.lellouche{at}criucpq.ulaval.ca
Dr Lellouche is a cofounder, shareholder, and administrator of Oxynov, a company that develops automated oxygen titration. Mr Branson discloses relationships with Inogen and Lung Pacer; he is Editor-in-Chief of Respiratory Care. Mr Bouchard has disclosed no conflicts of interest.
The study was performed at Institut Universitaire de Cardiologie et de Pneumologie de Québec.
A version of this paper was presented by Dr Lellouche at the SRLF meeting, held in Paris, France, June 12-14th, 2023.
- Copyright © 2024 by Daedalus Enterprises