Abstract
BACKGROUND: This study compared 3 nebulizer technologies for inter- and intradevice reproducibility, humidification, and fill volume sensitivity during mechanical ventilation: a breath-enhanced jet nebulizer, a vibrating mesh nebulizer, and a jet nebulizer. The breath-enhanced jet nebulizer featured a new design located on the wet side of the humidifier to reduce aerosol loss and potential humidifier contamination. The vibrating mesh nebulizer and the jet nebulizer were placed on the dry side.
METHODS: Aerosol delivery was measured using multiple ventilator settings (inspiratory time = 0.45–1.01 s). Using radiolabeled saline and a gamma camera, bench studies were performed using a ventilator to test 4 breathing patterns. Four scenarios were assessed during testing: 3 mL and 6 mL fill volumes with and without heated wire humidification. Measurements included inhaled mass (as a percentage of the nebulizer charge), nebulizer residual, mass balance, and aerosol particle size distribution. Statistics were determined using Mann-Whitney and linear regression.
RESULTS: The inhaled mass for the breath-enhanced jet nebulizer was 10.5–29.2% and was affected by fill volume (P = .004) but not by humidity. The inhaled mass for the vibrating mesh nebulizer was 0.9–33% and was unaffected by fill volume and humidity. The inhaled mass for the jet nebulizer was 2.5–25.9% and was affected by both fill volume (P = .009) and humidity (3 mL, P = .002). The inhaled mass for the vibrating mesh nebulizer was more variable due to random failures to achieve complete nebulization, and inhaled mass correlated closely with residual mass: IM% = –0.233(Residual%) + 24.3, r2 = 0.67, P < .001. For all devices, large particles were lost in the ventilator tubing; large particles were also lost in the humidifier for the vibrating mesh nebulizer (17% nebulizer charge), resulting in similar particle distributions (mass median aerodynamic diameter 1.33–1.95 μm) for all devices.
CONCLUSIONS: Nebulization with the breath-enhanced jet nebulizer was less sensitive to humidification than the jet nebulizer. Delivery via the vibrating mesh nebulizer was not predictable, with random failure to empty (55% experimental runs). All devices delivered similar particle distributions. Wet-side aerosol delivery avoids humidifier contamination, and breath-enhanced technology can ensure better control of drug delivery.
- aerosols
- nebulizers and vaporizers
- administration
- inhalation
- ventilators
- mechanical
- humidifiers
- drug delivery
- breath-enhanced
Introduction
Researchers have been studying aerosol delivery during mechanical ventilation for more than 30 years, yet there is no standard method for aerosol therapy. Uncontrolled variables include ventilator effects, such as bias flow,1,2 breath actuation settings,2-4 methods of humidification,2,5-8 nebulizer positioning,1,5,9-11 nebulizer technology,5-7,11,12 and device reproducibility.13 Many in vitro studies have tested these variables following ad hoc protocols, leading to an evolution of delivery techniques, especially changes in ventilators, their circuits, methods of humidification, and nebulizer technologies.
The present study was designed to assess current technologies over a range of practical settings and situations to provide a modern understanding of aerosol delivery during mechanical ventilation. Specifically, the protocol tested available technologies in terms of inter- and intradevice variability and sensitivity to humidification and volume-fill effects under real-use conditions. Three nebulizer technologies were tested: a novel breath-enhanced jet nebulizer called the i-AIRE (InspiRx, Somerset, New Jersey); a vibrating mesh nebulizer (Solo, Aerogen, Galway Ireland); and a jet nebulizer (Hudson MicroMist, Teleflex Medical, Morrisville, North Carolina). Nebulizer placement in the circuit was based on current practices which favor nebulizer location proximal to the ventilator.1,5,6,9,14,15 This position facilitates control of device orientation which may affect function. Effects of contemporary humidification systems were tested with each nebulizer technology over a range of ventilator settings and fill volumes to measure their performance and reliability. The vibrating mesh nebulizer and the jet nebulizer were located on the dry side of the humidifier1,5,15 (ie, on the humidifier inlet), whereas the breath-enhanced jet nebulizer was located on the wet side (ie, on the humidifier outlet). This study was performed at the Aerosol Laboratory in the Division of Pulmonary, Critical Care, and Sleep Medicine, in the Department of Medicine at Stony Brook University Medical Center, Stony Brook, New York.
Quick Look
Current Knowledge
During invasive ventilation, vibrating mesh nebulizers with low residual mass are reported to be more efficient than conventional jet nebulizers. Breath-enhanced jet nebulization is new for mechanical ventilation and has not been compared to existing technology. In addition, how these devices interact with other factors potentially affecting drug delivery (eg, active humidification, nebulizer fill volume, and day-to-day inter- and intradevice usage) is unknown.
What This Paper Contributes to Our Knowledge
Although vibrating mesh nebulizers may be highly efficient, they were unpredictable and widely variable in output. Conventional jet nebulizers were sensitive to fill volume and humidification, consistent with previously published studies. Using the breath-enhanced jet nebulizer on the wet side of the heated humidifier during mechanical ventilation can provide predictable and reliable drug delivery while avoiding effects of active humidification and humidifier contamination.
Methods
Experimental Setup
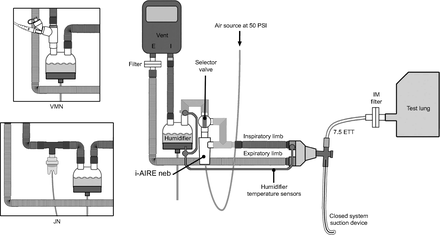
The experimental setups are illustrated in Figure 1 (humidified) and Figure 2 (nonhumidified). In Figure 1, the breath-enhanced jet nebulizer is shown in the circuit located on the wet side (outlet) of a conventional heated humidifier (ConchaTherm Neptune, Hudson RCI/Teleflex Medical, Morrisville North Carolina) set at 37°C. This position has been found to be more effective for this type of nebulizer technology.16 During treatment, the 2-way selector valve was turned, directing all inspiratory gas flow from the ventilator to flow through the top of the nebulizer and exit into the inspiratory limb of the circuit through the side port, thereby enhancing aerosol generation primarily during inspiration.16 Wall air at a flow of 3.5 L/min at 50 psi was turned on to power the nebulizer. The humidifier outlet temperature sensor regulating the humidifier was relocated from the standard location on the proximal end of the conventional inspiratory limb to the point where the 2-way selector valve was placed on the humidifier outlet. The circuit is designed so that when a treatment is completed, the gas flow powering the nebulizer is turned off and the selector valve is turned, bypassing the nebulizer and directing ventilator flow to the inspiratory limb of the circuit. The inset diagrams on Figure 1 depict the Aerogen vibrating mesh nebulizer and the Hudson jet nebulizer configurations, both placed on the dry side of the humidifier as recommended.15,17
Location of the i-AIRE breath-enhanced jet nebulizer connected to the heated humidifier at its outlet port (ie, the wet side) during active humidification. The selector valve may be turned so that inspiratory gas from the ventilator either bypasses the nebulizer or passes through it, for breath-enhancement, as gas flows to the patient. The inset diagrams show the vibrating mesh nebulizer and the jet nebulizer connected to the heated humidifier at its inlet port (ie, the dry side). VMN = vibrating mesh nebulizer; JN = jet nebulizer.
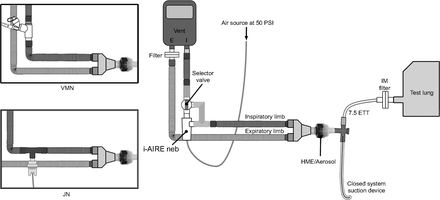
Although a heat-and-moisture exchanger (HME) was not used during this study, an aerosol bypass HME is shown to demonstrate its mounting location if used clinically. All nebulizers were placed ∼15 cm (∼6 inches) from the ventilator inspiratory limb outlet port. The i-AIRE breath-enhanced jet nebulizer with its selector valve was interposed between the ventilator outlet port and the inspiratory limb of the circuit. The inset diagrams show the vibrating mesh nebulizer and the jet nebulizer interposed into the inspiratory limb with their respective T-adapters. IM = inhaled mass; ETT = endotracheal tube; VMN = vibrating mesh nebulizer; JN = jet nebulizer.
Figure 2 shows the i-AIRE breath-enhanced jet nebulizer in the nonhumidified experimental setup. Although a heat-and-moisture exchanger (HME) was not used during this study, an aerosol bypass HME is shown to demonstrate its mounting location if used clinically. In this configuration, the breath-enhanced jet nebulizer, as well as the vibrating mesh nebulizer and jet nebulizer (shown in the inset diagram), were placed on the inspiratory limb ∼15 cm (∼6 inches) from the ventilator outlet, which has been described as an optimal position.1,5 The Hudson MicroMist jet nebulizer was attached to the ventilator circuit with a Hudson spring-loaded T-adapter and operated at a flow of 8 L/min as per manufacturer instructions using wall air at 50 psi. The Aerogen Solo vibrating mesh nebulizer was positioned in the circuit using the adult Aerogen T-adapter as per manufacturer specifications15 and operated via an Aerogen Pro-X Controller.
To duplicate typical hospital ventilator circuit setups, the patient Y-connector was attached to a closed-suction system device (Ballard Closed Suction System, Avanos Medical, Alpharetta, Georgia) and a 7.5-mm inner diameter endotracheal tube (Rusch, Teleflex Medical). The circuit was connected to a Training Test Lung (Michigan Instruments, Grand Rapids, Michigan) set with a resistance of 5 cm H2O and compliance of 40 mL/cm H2O. The Avea ventilator (Vyaire Medical, Mettawa, Illinois) was used with 4 different clinically relevant breathing patterns (Table 1) chosen to provide a range of ventilator duty cycles: 3 volume control modes with tidal volumes of 460–650 mL, and 1 pressure control mode with a pressure limit of 15 cm H2O. Frequencies of 15–20 breaths/min, PEEP of 5 cm H2O, and bias flow of 2.0 L/min at 21% oxygen was used for all settings. Nebulizers were operated continuously and were initially run to dryness (ie, cessation of visible aerosol output) using visual inspection. Run time was measured using a stopwatch.
Ventilator Settings
Inhaled Mass and Residual Activity
An inhaled mass (IM) filter (Pari, Starnberg, Germany) placed at the distal tip of the endotracheal tube collected the aerosol particles that would be inhaled by a patient under similar conditions. A similar filter was placed in the expiratory limb proximal to the exhalation channel for mass balance measurements. A total of 58 experiments were performed using 4 new devices of each nebulizer type. All nebulizers were rotated throughout the experimental protocol and tested a minimum of 4 times each for repeatability. Some nebulizers were tested more frequently because they were used for mass balance experiments. Each nebulizer was charged with either 3 mL or 6 mL of Technetium-99m radiolabeled saline. For all experiments, radioactivity placed in the nebulizers and radioactivity captured on the filters were measured with a gamma camera (Maxi Camera 400, General Electric, Horsholm, Denmark; Power Computing, Model 604/150/D, Austin, Texas; Nuclear Mac OS 4.2.2, Scientific Imaging, Thousand Oaks, California). At the conclusion of test runs, nebulizers were placed on the gamma camera to determine residual radioactivity, expressed as percent of nebulizer charge (Residual%).
Mass Balance
In a separate set of experiments, for each experimental configuration depicted in Figure 1 and Figure 2, a complete mass balance determination was carried out for each nebulizer type for the 3-mL fill volume. The radioactivity of aerosol deposited on circuit components was measured by placing each component separately on the gamma camera.
Aerosol Particle Size Distribution
Aerosol particle size distribution was determined via cascade impaction sampling at the distal tip of the endotracheal tube (Marple 8-stage impactor, Thermo Fischer Scientific, Waltham, Massachusetts) with a vacuum flow of 2.5 L/min. Distributions were determined for 2 samples of each nebulizer type using a 3-mL fill volume. Aerosols were sampled over a 3-min period. Radioactivity on the cascade stages was measured with a collimated ratemeter (Ludlum Measurements, Sweetwater, Texas), and the distribution was plotted on log probability paper to determine mass median aerodynamic diameter.18
Statistical Analysis
GraphPad Prism 8.3 for Mac OS (GraphPad Software, San Diego, California) was used to calculate mean ± SD for each nebulizer type with and without humidification and to generate log particle size versus probability graphs for aerosol particle size distribution. The Mann-Whitney test and linear regression analysis were used to assess inter- and intradevice variability. A P value < .05 was considered statistically significant.
Results
Aerosol delivery to the filter for each device, expressed as IM as a percent of nebulizer charge (IM%), is shown in Figure 3. Measurements of IM% for each experiment were plotted with IM% on the y axis against inspiratory time (TI) on the x axis, and different symbols were used to indicate humidification status and fill volume. Each data point represents 1 of the 4 devices for each nebulizer type as a function of ventilator setting (expressed as TI, a common variable for all settings), humidification, and fill volume. Statistical data for each configuration is tabulated in Table 2. Figure 3 shows that, for both the breath-enhanced jet nebulizer and the jet nebulizer, there was a gradual increase in delivery with increasing TI, which suggests some sensitivity to increasing duty cycle. The vibrating mesh nebulizer’s delivery did not appear to be affected by TI with more scatter over the range of ventilator settings. Mass balance data are tabulated in Table 3.
Inhaled mass versus inspiratory time (TI) for A: breath-enhanced nebulizer, B: vibrating mesh nebulizer, and C: jet nebulizer.
Inhaled Mass Effects of Humidification and Fill Volume
Mass Balance-3 mL Fill Volume
Breath-Enhanced Jet Nebulizer
IM% was significantly affected by fill volume, but only when humidified: 12.2 ± 6.2% for 3-mL fill volume and 23.9 ± 4.0% for 6-mL fill volume (P = .004). Humidifcation effects were not significant. For the 3-mL volume fill, the mass balance determination indicated that the nebulizer Residual% was decreased (Table 3) and circuit losses were higher with humidification, resulting in small insignificant changes in IM% with humidity. Mean run times for the 3-mL and 6-mL fill volume were 8 and 18 min, respectively.
Vibrating Mesh Nebulizer
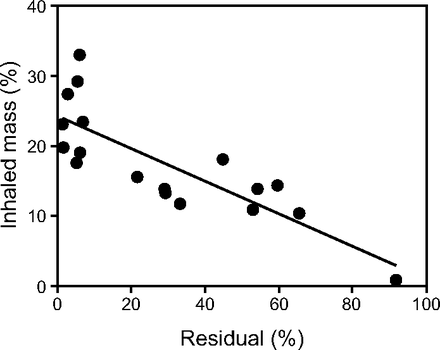
Humidity and fill volume had no significant effects on IM%. Visually, however, data for the vibrating mesh nebulizer varied over a wider range than the other devices (Fig. 3). Some of this variability was related to variables intrinsic to the nebulizer (ie, separate from the conditions of the ventilator circuit). The factors influencing vibrating mesh nebulizer function were assessed in Figure 4, which is a plot of IM% against nebulizer Residual%. Some devices emptied nearly completely with Residual% being < 10% (left side of Fig. 4), close to the small Residual% expected for this technology. However, for all the data, variation in IM% was closely correlated with wide variation in the nebulizer Residual%. Overall, the Solo failed to empty 55% of the time (ie, 10 of 18 runs). Failure to empty occurred randomly; for example, a device would empty completely on one run and fail to empty on the next run. When the nebulizer functioned properly (ie, with a low Residual%), the mass balance determination (Table 3) demonstrated a loss of 17% in the humidifier during humidification and 26% in the vent circuit. In the nonhumidified circuit, 48.6% was lost. The average run times for 3-mL and 6-mL fill volumes were 11 min and 20 min, respectively.
Inhaled mass versus nebulizer residual for vibrating mesh nebulizer experiments: inhaled mass = −0.233 (Residual%) + 24.3, r2 = 50.67, P < .001.
Jet Nebulizer
IM% was strongly affected by fill volume, increasing 2-fold regardless of humidity: 5.5 ± 1.6% for 3 mL and 16.3 ± 5.7% for 6 mL (P = .009) when actively humidified versus 10.9 ± 2.4% for 3 mL and 18.8 ± 8.8% for 6 mL for nonhumidified (P = .007). For a given fill volume, significant humidification effects were seen only for the 3-mL fill volume: 5.5 ± 1.6% humidified compared to 10.9 ± 2.6% for nonhumidified (P = .002). The mass balance determination (Table 3) indicated minute losses in the humidifier of 0.1%, with nebulizer Residual% as high as 70%. Average run times for 3-mL and 6-mL fill volumes were 6 and 17 min, respectively.
Aerosol Particle Size Distribution
Aerosol particle size distributions at the distal tip of the endotracheal tube were similar for all 3 devices (Figure 5). The mean ± SD of 2 samples of each nebulizer type, with a 3-mL fill volume, with and without humidification, were 1.95 ± 0.21 µm for the humidified breath-enhanced jet nebulizer; 1.45 ± 0.01 µm for the nonhumidified breath-enhanced jet nebulizer; 1.90 ± 0.14 µm for the humidified vibrating mesh nebulizer; 1.57 ± 0.05 µm for the nonhumidified vibrating mesh nebulizer; 1.55 ± 0.07 µm for the humidified jet nebulizer; and 1.33 ± 0.03 µm for the nonhumidified jet nebulizer.
Aerosol particle size distributions for 3 nebulizer types, with and without heated humidification. The test breathing pattern consisted of volume control continuous mechanical ventilation, frequency = 18 breaths/min, tidal volume = 500 mL, inspiratory flow = 45 L/min, inspiratory time = 0.7 s, PEEP = 5 cm H2O, and bias flow = 2.0 L/min). Log particle size for each cascade stage plotted against probability. The following values are presented as mean ± SD at 50% probability: mass median aerodynamic diameter: breath-enhanced jet nebulizer = 1.95 ± 0.21 µm (humidified), 1.45 ± 0.01 µm (nonhumidified); vibrating mesh nebulizer = 1.90 ± 0.14 µm (humidified), 1.57 ± 0.05 µm (nonhumidified); jet nebulizer = 1.55 ± 0.07 µm (humidified), 1.33 ± 0.03 µm (nonhumidified).
Discussion
Aerosol delivery to the ventilated patient is continuously evolving. In this study, we tested representative samples of 3 current nebulizer technologies over a range of settings with and without humidification using fill volumes typical for common drugs. We tested multiple examples of each device to mimic real-world usage, as we sought to document both inter- and intradevice variability. Common drugs such as bronchodilators that are used off-label on ventilators were originally approved for hand-held nebulization. Their clinical effectiveness is often monitored at the bedside because the dose response of these devices cannot be predicted for the individual patient. It is reasonable to assume that knowledge of a device’s performance characteristics will enhance the caregiver’s assessment of a therapy’s effectiveness. The jet nebulizers tested in this study were powered from wall air to control delivery conditions because modern ventilators do not have standardized jet nebulizer driving systems, and in some cases they do not support jet nebulization at all.
On average, the breath-enhanced jet nebulizers and the vibrating mesh nebulizers demonstrated equal overall delivery, with both devices more efficient than the conventional jet nebulizers. Vibrating mesh nebulizer delivery, however, was less predictable, with delivery ranging between 0.9% and 33% in individual experiments. The vibrating mesh nebulizer behaved differently than the jet nebulizer devices, exhibiting greater variation in IM%. Figure 4 displays the relationship between the IM% and Residual%. The residual mass for each run was expected to be a low percentage (ie, < 10%). There were 2 populations of Residual% values: one group was clustered at low values, and the other was randomly elevated. The former represents intrinsic differences between the devices, perhaps due to membrane function or other uncontrolled factors; the latter represents failure of the device to empty. Because each example of the Solo tested was rotated between experiments, the failure to empty was not a hard failure, that is, not an electrical or mesh issue, rather a random failure to empty. The same device that stopped nebulizing on a given run functioned normally on a repeat run. This observation is not a mesh defect but is likely related to failure of the liquid stream from the reservoir to contact the mesh. This behavior has been described previously for isolated devices tested during continuous nebulization with fill volumes of 3 mL.13 For the jet nebulizers in this study, the Residual% was relatively reproducible for each condition.
There were important differences between devices when used with humidification. Aerosol delivery for the newer technologies was less sensitive to humidification. Effects on vibrating mesh nebulizer aerosol delivery were not detectable, although the dependence of delivery on device emptying may have obscured potential effects of humidification. For the breath-enhanced jet nebulizer, compared to nonhumidified delivery, wet side nebulization had a lower Residual%. This unique observation was first reported for this technology by Cuccia et al,16 who studied a breath-actuated version of the i-AIRE breath-enhanced jet nebulizer. Contrary to conventional jet nebulization, where expected reductions in aerosol delivery can approach 50%,6 Cuccia et al16 reported that wet side nebulization with the i-AIRE resulted in preserved overall delivery with humidification. In both the study by Cuccia et al16 and the present study, the decreased Residual% was matched by increased losses in the ventilator tubing resulting in similar aerosol delivery between dry and wet circuits (Table 3).
Cuccia et al16 used the i-AIRE in a breath-actuated mode in which the 3.5-L/min air flow powering the nebulizer was interrupted during expiration by a computer-driven solenoid valve. Under those conditions, the authors reported an IM% of 31.1 ± 6.33%, approximately double that seen in our results. In other words, their breath-actuated device avoided aerosol losses during expiration. In this study, the i-AIRE in continuous operation (without the solenoid trigger) is compared with other devices currently in use for routine aerosol therapy (eg, bronchodilators).
The conventional jet nebulizer behaved as expected for this technology.2 Specifically, IM% was significantly reduced with humidity at 3 mL volume fill. This effect was first reported on the bench in 1992 by O’Riordan et al,6 with in vivo confirmation by Miller et al,2 who reported that active humidification significantly reduced delivery of inhaled antibiotics to intubated patients. Those data led Palmer and colleagues19-21 to design clinical protocols to test effects of inhaled antibiotics delivered while deliberately avoiding circuit humidification during active nebulization. Our results indicate that wet side nebulization with the i-AIRE breath-enhanced jet nebulizer avoids the adverse effects on aerosol delivery and ensure that clinicians can expect equal efficiency of delivery with or without active humidification. Both the i-AIRE breath-enhanced jet nebulizer and the Hudson jet nebulizer were sensitive to changes in nebulizer fill volume, showing significant increases in efficiency with increased volume. For the Hudson jet nebulizer, the Residual% was the highest of all tested devices, and delivery was relatively low under all conditions unless the nebulizer charge was increased to 6 mL. The increase in IM% with a higher fill volume is a known factor for jet nebulizers during spontaneous breathing; the higher the fill volume, the greater the output.2,12,22,23 Overall, the Aerogen vibrating mesh nebulizer was most efficient with the lowest Residual%, but those gains were largely canceled by repeated failure of the vibrating mesh nebulizer to empty.13
In spite of its dry-side location, the Hudson jet nebulizer did not deposit particles in the humidifier, whereas the Aerogen vibrating mesh nebulizer deposited 17% of the dose. The differences in behavior between jet and mesh devices may be due to the 8 L/min of dry air flow used to drive the jet nebulizer. The high gas flow for the jet nebulizer may affect local particle size such that the largest particles are immediately reduced in size, thus allowing aerosol to pass through the humidifier without depositing.
Respiratory therapists are aware that expiratory volume readouts on the ventilator are commonly affected during jet nebulization. However, from previous studies in our laboratory, jet flow ranging from 3.5 to 10 L/min had minimal effect on delivered volume.16,24
Finally, our data indicate that, independent of the technology used, the aerosol particle size distributions measured at the distal tip of the endotracheal tube were similar for all 3 devices, with a mass median aerodynamic diameter of 1.33–1.95 µm, demonstrating that large particles are lost in the circuit.
Conclusions
When nebulizing medication during conventional mechanical ventilation, there are important differences in drug delivery between commercially available delivery devices that are independent of the ventilator. Knowledge of these differences could account for clinical differences in response.
Acknowledgments
The authors thank Lorraine Morra for her time and assistance with technical and statistical analysis. We also appreciate the loan of equipment and supplies from the Respiratory Care Department at Stony Brook University.
Footnotes
- Correspondence: Sunya Ashraf MD. E-mail: sunya.ashraf{at}stonybrookmedicine.edu
See the Related Editorial on Page 1624
A version of this paper was presented at the Open Forum of the AARC Congress 2019, held November 9–12, 2019, in New Orleans, Louisiana.
This study was supported in part by InspiRx (Somerset, New Jersey). Dr Smaldone and Ms Cuccia have disclosed relationships with InspiRx Dr Ashraf and Mr McPeck disclosed no conflicts of interest.
- Copyright © 2020 by Daedalus Enterprises