Abstract
BACKGROUND: Weaning through noninvasive ventilation (NIV) after early extubation may facilitate invasive ventilation withdrawal and reduce related complications in patients with hypercapnic respiratory failure. However, the effects of NIV weaning are uncertain in patients with acute hypoxemic respiratory failure (AHRF). We aimed to investigate whether NIV weaning could reduce hospital mortality and other outcomes compared with invasive weaning in subjects with hypoxemic AHRF.
METHODS: We searched medical literature databases for relevant articles published from inception to February 2019. Randomized controlled trials that adopted NIV as a weaning strategy compared with invasive weaning in hypoxemic AHRF were included. The primary outcome was hospital mortality. The secondary outcomes included ICU mortality, the ICU stay, weaning time, duration of ventilation, extubation failure, and adverse events.
RESULTS: Six relevant studies, which involved 718 subjects, were included. There was no significant effect of NIV weaning on hospital mortality compared with invasive weaning (risk ratio 0.94, 95% CI 0.65–1.36; P = .74), whereas there was a significant effect of NIV weaning on shortening the ICU stay (mean difference −3.95, 95% CI −6.49 to −1.40, P = .002) and on decreasing adverse events without affecting the weaning time (standardized MD −0.04, 95% CI −0.21 to 0.14; P = .68).
CONCLUSIONS: The strategy of NIV weaning did not decrease hospital mortality in subjects with hypoxemic AHRF, but it did shorten the ICU lengths of stay and reduce adverse events.
- noninvasive ventilation
- invasive ventilation
- weaning
- hypoxemic respiratory failure
- systematic review
- meta-analysis
Introduction
As a vital lifesaving method, invasive mechanical ventilation is important to patients with acute hypoxemic respiratory failure (AHRF). However, prolonged ventilation is associated with increased complications, morbidity, and mortality.1,2 A previous study reported that the time spent in the weaning process accounted for 40–50% of the duration of total mechanical ventilation and was the main cause of prolonged ventilation.3 At present, how to optimally wean from invasive ventilation optimally has attracted much attention. Currently, the recommendations to facilitate liberation from invasive ventilation include spontaneous breathing trials (SBT), protocols to minimize sedation, and preventive noninvasive ventilation (NIV).4 Although most patients can eventually be successfully weaned from invasive ventilation, one third of patients still require more than one SBT and are considered difficult to wean.5,6
Recently, it was revealed that NIV weaning might serve to liberate patients early who had had weaning difficulty.3,7 NIV weaning might decrease hospital mortality and reduce the duration of invasive ventilation and the incidence of ventilator-associated pneumonia in patients with COPD who were hypercapnic or in a mixed population (ie, patients with hypercapnia and hypoxemia of various etiologies).8-11 To our knowledge, the characteristics of patients with hypoxemic AHRF differ from those of patients with hypercapnic AHRF in terms of clinical features, pathophysiologic mechanisms, and prognosis. Nevertheless, presently, the effect of NIV weaning on the outcomes of hypoxemic AHRF is unclear. Therefore, we aimed to investigate the efficacy of NIV weaning on hospital mortality, ICU stay, adverse events, and other outcomes compared with invasive weaning in subjects with hypoxemic AHRF.
Methods
Ethics approval and patient consent were not required for this meta-analysis. We conducted this systematic review and meta-analysis in accordance with the published guidelines12 and compliance with the PRISMA statement registered in PROSPERO (CRD42019125519).
Search Strategy
We searched MEDLINE, Embase, the Cochrane Library, and Ovid bibliographic databases from inception to February 2019 without language restrictions. The selection of literature in Chinese was limited to papers published in Chinese Core Journals to ensure the quality of the study. The search strategy used a combination of key words and MeSH (Medical Subject Headings) terms as follows: “noninvasive ventilation,” “respiratory insufficiency,” “respiratory failure,” “respiration, artificial” and “ventilator weaning.” Additional references of all the included studies were identified through citation tracking. Two authors (MS, XP) independently performed the search and excluded irrelevant titles. Duplicate articles were identified and removed. Disagreements were resolved by discussion and arbitration by a third author (WW).
Eligibility Criteria
All relevant studies that adopted NIV as a weaning method were screened and evaluated. The following inclusion criteria: (1) randomized controlled trials that compared NIV weaning with invasive weaning; (2) adults (>18 years old) with hypoxemic AHRF or a mixed population with <5% of subjects with COPD; (3) intubated and invasive ventilation for >24 hours; and (4) studies that reported at least one of the following mortality, ICU stay (length of stay [LOS]), extubation failure, duration of ventilation, weaning time, and adverse events. The exclusion criteria were the following: (1) studies that only included subjects with hypercapnic AHRF caused by COPD exacerbation or included ≥5% subjects with COPD in a mixed population; (2) subjects ≤ 18 years old; (3) weaning during the first 24 hours of ventilation; (4) studies that compared NIV weaning with standard oxygen therapy after ventilator weaning; (5) studies that used NIV as the rescue or preventive therapy after extubation; (6) studies with animals; (7) studies only published as meeting papers or abstracts without full texts; and (8) case reports, reviews, and editorials.
Data Extraction
Data were extracted from the qualified articles by two of us (MS, XP). Disagreements were resolved by discussion and arbitration with one author (WW). The extracted data included study characteristics (publication year, inclusion criteria, sample size), population characteristics (the causes of NIV in the included patients), and a strategy for the intervention and comparator. Hospital mortality was considered the primary outcome. For the secondary outcomes, ICU mortality, the rate of extubation failure, the duration of total ventilation and invasive ventilation, weaning time, the ICU LOS, and adverse events were extracted. Extubation failure was defined as the inability to sustain spontaneous unassisted breathing and the need for NIV or re-intubation within 48 hours after extubation.
Risk of Bias Assessment
The Cochrane tool12 was used to assess the possible risk of bias in the randomized controlled trials. This tool covered domains that included sequence generation, allocation concealment, blinding of participants and personnel, blinding of the outcome assessment, incomplete outcome data, selective reporting, and other biases. Assessments were undertaken by two of us (MS, XP) independently.
Statistical Analysis
We pooled the dichotomous and continuous data by using risk ratios (RR) and mean differences (MD) or standardized MDs with 95% CIs as summary estimates of the effect. Heterogeneity across the studies was tested by using the Q statistic and I2 test.13 I2 values of 25–50% indicated low, 50–70% moderate, and >75% high heterogeneity.14,15 If moderate or high heterogeneity was present, then sensitivity analyses were conducted to determine the source of the heterogeneity. Fixed-effects models or random-effects models were adopted to analyze the pooled data according to the heterogeneity. The primary outcome of mortality was calculated by the Mantel-Haenszel method in a fixed-effects model because of the low heterogeneity. Potential publication bias on hospital mortality was estimated by constructing and visually inspecting a funnel plot in which the log RRs were plotted against their standard errors. Statistical analyses were performed with Review Manager 5.3 software (Cochrane Collaboration, Oxford, United Kingdom). We adopted Egger tests to further evaluate publication bias by using Stata 14.0 (StataCorp, College Station, Texas).
Results
Study Selection and Identification
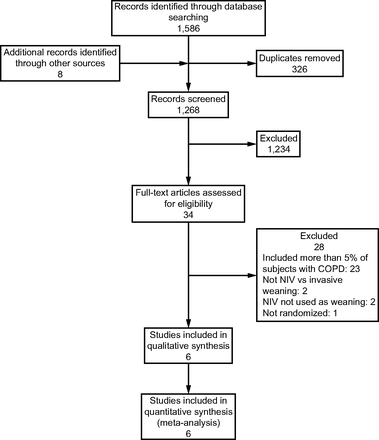
The literature review identified 1,586 records in the databases, and 8 additional studies were added through citation tracking (Fig. 1). After removal of the duplicates, 1,268 studies were retrieved to be reviewed. By screening the titles and abstracts, 1,234 irrelevant studies were excluded. Then, the full texts were assessed in greater detail. Finally, 6 randomized controlled trials,16-21 which involved 718 participants, were included in the meta-analysis. These studies were conducted in 4 different countries: Italy,16 Pakistan,17 China,18,19 and the United Kingdom.20 One study21was conducted in 9 centers in Italy and China. One study20 was conducted in 41 centers, and the other studies16-19 were in single centers. Five studies16-19,21 recruited hypoxemic subjects without COPD. In one study,20 <3.9% of the subjects had COPD. Three studies17,19,20 (n = 515 [72%]) used failed SBT as an inclusion criterion for study entry. One study18 (n = 53 [7%]) did not describe the weaning protocol in detail. The characteristics of the included studies are shown in Table 1.
Flow chart. NIV = noninvasive ventilation.
Characteristics of Included Studies
Risk of Bias Assessment
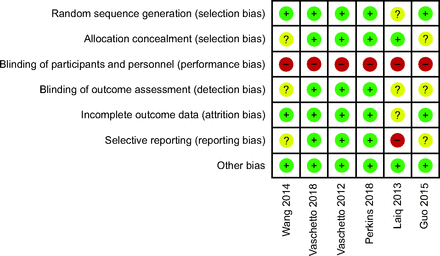
Overall, the included studies were of moderate-to-high quality (Fig. 2). The majority of the studies described the method of randomization and allocation concealment. It was impossible to blind the participants and personnel to treatment allocation, which meant that all included studies were considered to be high risk for performance bias. A few studies were not registered, which might have created a risk of selective reporting.
Risk of bias.
Effect of NIV Weaning on Mortality
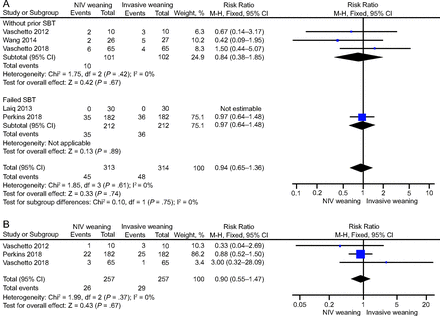
Five studies,16-18,20,21 which recruited 627 subjects, reported hospital mortality (RR 0.94, 95% CI 0.65–1.36; P = .74), with no statistical heterogeneity (I2 = 0%, P = .61), which was 14.38% in the NIV weaning group versus 15.29% in the invasive weaning group; the pooled results showed no significant difference in hospital mortality between the groups (Fig. 3A). ICU mortality was extracted from 3 studies;16,20,21 the pooled data showed no significant effect on ICU mortality (RR 0.90, 95% CI 0.55–1.47, P = .67) (Fig. 3B). One study19 provided 60 days of mortality data.
Effect of NIV weaning on (A) hospital mortality and (B) ICU mortality. SBT = spontaneous breathing test, NIV = noninvasive ventilation.
Effect of NIV Weaning on ICU LOS and Weaning Time
As shown in Figure 4A, ICU LOS was evaluated in 5 studies,16-18,20,21 which recruited 627 subjects. The pooled data showed that NIV weaning dramatically shortened the ICU LOS compared with invasive weaning (MD –3.95, 95% CI –6.49 to –1.40; P = .002), whereas the heterogeneity was high (I2 = 90%). A further sensitivity analysis indicated that the heterogeneity originated from the quality of 2 studies.17,18 Subgroup analysis according to the quality of the studies showed that NIV weaning shortened the ICU LOS in both the high-quality subgroup (MD –1.23, 95% CI –2.38 to –0.08, P = .04), with low heterogeneity (I2 = 0%), and the moderate-quality subgroup (MD –6.98, 95% CI –12.34 to –1.61, P = .01), with high heterogeneity (I2 = 90%) (Fig. 4B). The subgroup analysis according to the etiology of diseases showed that NIV weaning significantly shortened the ICU LOS in the surgical population (MD –5.20, 95% CI –7.96 to –2.44, P < .001), whereas it did not shorten the ICU LOS in the medical or the mixed population (Fig. 4C). Three studies16,20,21 that involved 514 participants reported weaning time. The pooled results showed that there was no difference in weaning time between the 2 groups (Fig. 4D).
Effect of noninvasive ventilation (NIV) weaning on (A–C) ICU LOS and (D) weaning time.
Effect of NIV Weaning on the Rate of Adverse Events
For adverse events, the pooled data demonstrated a beneficial effect of NIV weaning on reducing the rates of ventilator-associated pneumonia (RR 0.44, 95% CI 0.28–0.70; P < .001). The use of sedatives was reported in 3 studies,16,20,21 which included 514 subjects. The studies reported that the sedation use of the NIV weaning group was lower than that of the invasive weaning group (RR 0.85, 95% CI 0.73–0.98; P = .030). The rates of tracheostomy in the NIV weaning group were less than that in the invasive weaning group (RR 0.70, 95% CI 0.50–0.98; P = .040). Moreover, the re-intubation rates in the NIV weaning group were increased (RR 1.45, 95% CI 1.11–1.90, P = .007). Detailed information on the adverse events is shown in Table 2.
Summary Estimates of Effect of Noninvasive Ventilation on Adverse Events and/or Tracheostomy
Effect of NIV Weaning on Other Outcomes
Data on the duration of total ventilation was obtained from 2 studies18,19 (see the supplementary materials at http://www.rcjournal.com). Pooled data showed that NIV weaning reduced the duration of total ventilation (standardized MD –0.46, 95% CI –0.79 to –0.12; P = .007). Extubation failure could not be synthesized because it was reported in limited studies and the definitions differed.
Subgroup Analysis
We conducted a subgroup analysis by clinical heterogeneity to evaluate studies that enrolled subjects without previous SBT versus failed SBT (Fig. 3A). We noted a nonsignificant effect of NIV weaning on hospital mortality between the subgroups. Subgroup analysis of studies by previous SBT showed that the pooled ICU LOS was shorter in the NIV weaning strategy compared with the invasive weaning strategy in patients with failed SBT (MD –3.07, 95% CI –6.10 to –0.04; P = .050) (Fig. 4A). We performed subgroup analysis further according to the method of NIV interfaces. Subgroup analyses of the NIV interfaces found that the interfaces did not affect the primary outcome, whereas the face mask shortened the ICU LOS compared with the other interfaces (Table 3).
Subgroup Analysis of the Interfaces Used in the Experimental Group
Publication Bias
Visual inspection of a funnel plot (Fig. 5) and the Egger test (P = .031) indicated a little asymmetry in the effect of NIV weaning on hospital mortality.
Funnel plots to assess publication bias with regard to the effect of noninvasive ventilation weaning on hospital mortality.
Discussion
To our knowledge, this was the first qualitative synthesis to investigate the effect of NIV weaning in subjects with hypoxemic AHRF. The meta-analysis indicated that NIV weaning did not decrease hospital mortality compared with invasive weaning, whereas it did significantly shorten the ICU LOS and decrease the incidence of adverse events. Results of a number of studies indicated that the use of NIV reduced the need for endotracheal intubation and associated complications in selected subjects with AHRF.22-26 In recent years, NIV has been tentatively adopted as a weaning modality.21,22,27 NIV weaning reduced hospital mortality and rates of complications in subjects with AHRF, but this effect was predominantly in subjects with an exacerbation of COPD.10,11
To our knowledge, NIV has been used effectively and extensively in patients with AHRF caused by the exacerbation of COPD.24,28,29 These patients might be ideally suited for NIV weaning because of the pathophysiologic mechanisms.30,31 The overall benefit of NIV weaning might be overstated in numerous studies by contamination with a population composed predominantly of subjects with COPD. In contrast, a large randomized controlled trial showed no significant effect of NIV weaning on hospital mortality and ICU LOS compared with invasive weaning in subjects with hypoxemic AHRF.20 As a result, no consensus has been reached on the effect of NIV weaning in this kind of patient.
This meta-analysis showed that NIV weaning did not reduce hospital mortality in subjects with hypoxemic AHRF compared with invasive weaning. It differed from the results of previous reviews that involved subjects with COPD.10,11,32 This difference may be associated with the different mechanisms of respiratory failure. The genesis of hypoxemic AHRF is related to atelectasis, collapsed alveoli, retained secretions, and hypoventilation.33 To our knowledge, respiratory failure caused by this pathogenesis usually responds poorly to NIV.33,34
In addition, the etiologic causes of hypoxemic AHRF, such as ARDS, pneumonia, postoperative AHRF, and thoracic trauma, might impact the outcomes of those selected patients.35 Previous studies demonstrated that subjects with ARDS did not benefit from NIV.36,37 In patients with pneumonia, NIV has been frequently used but has been associated with a high rate of failure.33,38,39 For patients with ARDS or severe pneumonia, adopting NIV as a weaning strategy might tend to increase poor outcomes.40 An exciting finding of this subgroup analysis was that surgical subjects were more likely to benefit from NIV weaning than those with medical diseases. NIV weaning significantly shortened the ICU LOS in the surgical population. This finding was consistent with the results of research that involved subjects with trauma or postoperative AHRF, which showed that these subjects responded favorably to NIV.41-44
The pooled results of this review demonstrated that NIV weaning shortened the ICU LOS, predominantly in subjects with a failed SBT. Patients with a failed SBT are likely to develop a rapid and shallow breathing pattern.45 One study reported that NIV reduced the work of breathing and improved hypercapnia, hypoxemia, and patient-ventilator synchrony,46 and might further reduce the rate of extubation failure and shorten the duration of ventilation and the ICU LOS. Three studies included in our review indicated that the number of days of invasive ventilation was significantly reduced in the NIV weaning strategy, although the data could not be pooled due to the use of different definitions.16,18,21 Many factors, including the different quality of the studies and the different interfaces, might affect the ICU LOS. In our subgroup analysis by study quality, NIV weaning significantly shortened the ICU LOS, both in the high-quality studies16,20,21 and the moderate-quality studies.17,18 The subgroup analyses according to the NIV interfaces found that the face mask might shorten the ICU LOS compared with other interfaces, although the interfaces did not affect the mortality rate.
In addition, less sedation and excellent tolerance to NIV weaning could reduce the incidence of complications.47-49 As shown in this study, the rate of ventilator-associated pneumonia was 13.3% in the NIV weaning group and <30.9% in the invasive weaning group. The lower rates of complications and shorter duration of intubation shortened total ventilation time, although this strategy increased the re-intubation rate. Protocolized weaning was also related to shorter total ventilation and weaning times.50,51 However, this summary of pooled evidence showed no significant difference in weaning times between the 2 groups. It is understandable that NIV weaning allows earlier extubation, but it is not necessary to wean ventilatory support rapidly in patients with weaning difficulty.20,52,53
Several limitations should be acknowledged. First, the nature of the intervention prohibited clinicians and outcome assessors from blinding, which may have led to performance bias. Second, the ICU LOS might be affected by local habits, organizational protocols, post-ICU availability, and team expertise. Third, many factors, including different years of published studies and different ventilators, might affect the results to certain extents.
Conclusions
This systemic review indicated that NIV weaning did not decrease hospital mortality, but it significantly shortened the ICU LOS and decreased the rates of adverse events compared with invasive weaning in the subjects with hypoxemic AHRF with mixed diseases. Large randomized controlled trials are still needed to provide stronger evidence for specified diseases.
Acknowledgments
We thank the staff of the Chinese Evidence-Based Medicine Center for their training on systematic review methods. In addition, we thank the Chinese Evidence-Based Medicine Center, West China Hospital, and Sichuan University for providing the Stata 14.0 statistical software.
Footnotes
- Correspondence: Qi Liu MD, the First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan Province 450052, China. E-mail: qi.liu{at}vip.163.com or Changju Zhu MD, the First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan Province 450052, China. E-mail: zhuchangju98{at}163.com
This work was supported by the National Natural Science Foundation of China (grant 81400051) and the Key Project of Henan Higher Education (grant 19A320073).
The authors have disclosed no conflicts of interest.
Registered in PROSPERO (CRD42019125519).
Supplementary material related to this paper is available at http://www.rcjournal.com.
- Copyright © 2020 by Daedalus Enterprises