Abstract
BACKGROUND: Transcutaneous measurements of CO2 and O2 ( ,
, 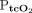 ) are noninvasive and allow for continuous monitoring in adults with exacerbation of COPD, but substantial accuracy issues may exist. We investigated agreement between results of arterial blood gas analysis and transcutaneous measurements of CO2 and O2 in patients with COPD.
) are noninvasive and allow for continuous monitoring in adults with exacerbation of COPD, but substantial accuracy issues may exist. We investigated agreement between results of arterial blood gas analysis and transcutaneous measurements of CO2 and O2 in patients with COPD.
METHODS: Adult subjects were monitored after acute admission to a respiratory intermediate care unit or ICU due to exacerbation of COPD and with ongoing noninvasive ventilation or immediately following extubation. Monitored variables were continuous transcutaneous measurement and simultaneous routine arterial blood gas analysis. Agreement between measurements was assessed by calculating bias with 95% limits of agreement for single-point estimates of 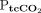 versus
versus  and versus
and versus 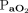 , and for changes in transcutaneous measurements between 2 time points (
, and for changes in transcutaneous measurements between 2 time points (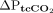 and
and 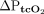 ). We considered limits of agreement within ± 7.5 mm Hg to be acceptable.
). We considered limits of agreement within ± 7.5 mm Hg to be acceptable.
RESULTS: A total of 57 transcutaneous measurements were made in 20 subjects for comparison with concurrent arterial blood gas analysis at 36 time points. The bias (limits of agreement) for 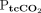 and
and 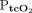 was 2.5 mm Hg (–10.6 to 15.6 mm Hg) and 11.2 mm Hg (–28.2 to 50.6 mm Hg), respectively. The bias for
was 2.5 mm Hg (–10.6 to 15.6 mm Hg) and 11.2 mm Hg (–28.2 to 50.6 mm Hg), respectively. The bias for 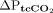 and
and  was 2.3 mm Hg (–3.8 to 8.3 mm Hg) and –5.3 mm Hg (–37.5 to 27 mm Hg), respectively.
was 2.3 mm Hg (–3.8 to 8.3 mm Hg) and –5.3 mm Hg (–37.5 to 27 mm Hg), respectively.
CONCLUSIONS:  and
and 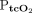 did not accurately reflect results from arterial blood gas analyses in this study of mostly hypercapnic subjects. Agreement between changes in CO2 during the monitoring period was acceptable, however, and transcutaneous monitoring may be used for continuous monitoring of
did not accurately reflect results from arterial blood gas analyses in this study of mostly hypercapnic subjects. Agreement between changes in CO2 during the monitoring period was acceptable, however, and transcutaneous monitoring may be used for continuous monitoring of  in conjunction with arterial blood gas analysis for reference.
in conjunction with arterial blood gas analysis for reference.
- transcutaneous blood gas monitoring
- noninvasive ventilation
- COPD
- hypercapnia
- respiratory insufficiency
- intensive care
Introduction
Arterial blood gas analyses (ABGs) are frequently performed in patients with exacerbation of COPD. Noninvasive transcutaneous monitoring of CO2 and O2 partial pressure (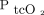 and
and 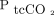 ) hold promising potential in terms of reducing the need for arterial punctures and thereby reducing the risks and discomfort related to this procedure.1 Moreover, transcutaneous monitoring allows for continuous measurement as an indicator of blood gas staus and may be particularly useful in clinical settings where frequent assessments of oxygenation and ventilation are needed, including during noninvasive ventilation (NIV).2 As approximately 12% of patients hospitalized with exacerbation of COPD require NIV, the scope of clinical use is substantial.3,4 Furthermore, arterial blood sample procedures are potentially painful.5 In 1 study, up to 50% of subjects reported pain > 5 on a scale of 10 in numeric ranked pain, and with > 1 puncture on average required to obtain a blood sample,6 a noninvasive technique to reduce the puncture frequency is obviously directly beneficial. Although rare, each puncture carries with it the risk of infection, and not so rarely a hematoma develops.7
) hold promising potential in terms of reducing the need for arterial punctures and thereby reducing the risks and discomfort related to this procedure.1 Moreover, transcutaneous monitoring allows for continuous measurement as an indicator of blood gas staus and may be particularly useful in clinical settings where frequent assessments of oxygenation and ventilation are needed, including during noninvasive ventilation (NIV).2 As approximately 12% of patients hospitalized with exacerbation of COPD require NIV, the scope of clinical use is substantial.3,4 Furthermore, arterial blood sample procedures are potentially painful.5 In 1 study, up to 50% of subjects reported pain > 5 on a scale of 10 in numeric ranked pain, and with > 1 puncture on average required to obtain a blood sample,6 a noninvasive technique to reduce the puncture frequency is obviously directly beneficial. Although rare, each puncture carries with it the risk of infection, and not so rarely a hematoma develops.7
The sensor for transcutaneous monitoring was invented over 50 years ago.8 It heats the skin to increase blood flow and arterialize the blood while increasing permeability for diffusion of blood gases.9 According to the American Association for Respiratory Care clinical practice guideline,10 the technology is currently applicable in various settings, including sleep investigations, during mechanical ventilation, and in evaluation of tissue perfusion, but a recent systematic review found substantial differences between 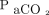 and
and 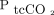 measurements depending on sensor placement site and temperature and the clinical indication for use.11 Some studies suggest that the accuracy of
measurements depending on sensor placement site and temperature and the clinical indication for use.11 Some studies suggest that the accuracy of  may decrease at higher levels of
may decrease at higher levels of 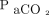 , but these reports are conflicting.12-14 The trending ability of transcutaneous monitoring may be useful in clinical practice, but further studies of this application are needed.11
, but these reports are conflicting.12-14 The trending ability of transcutaneous monitoring may be useful in clinical practice, but further studies of this application are needed.11
The aim of this study was to assess agreement between single-point estimates of ABGs and transcutaneous measurements in patients with exacerbation of COPD during NIV treatment or immediately after extubation. We hypothesized that 95% limits of agreement (LoA) between the 2 methods for both  and
and 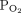 would be within ± 7.5 mm Hg. We also aimed to evaluate agreement between the 2 methods’ ability to reflect changes in the blood gases, hypothesizing that 95% LoA were within ± 7.5 mm Hg. We further compared agreement with ABGs at 2 different placements sites of the sensor, hypothesizing that there would be acceptable agreement at both sites.
would be within ± 7.5 mm Hg. We also aimed to evaluate agreement between the 2 methods’ ability to reflect changes in the blood gases, hypothesizing that 95% LoA were within ± 7.5 mm Hg. We further compared agreement with ABGs at 2 different placements sites of the sensor, hypothesizing that there would be acceptable agreement at both sites.
Quick Look
Current Knowledge
Transcutaneous monitoring of  and
and 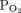 has been used in neonates for years, but agreement between arterial values and transcutaneous values has not been as good in adults. Recent studies have reported contrasting results regarding agreement in adult subjects and some studies exhibited larger discrepancies when subjects were hypercapnic.
has been used in neonates for years, but agreement between arterial values and transcutaneous values has not been as good in adults. Recent studies have reported contrasting results regarding agreement in adult subjects and some studies exhibited larger discrepancies when subjects were hypercapnic.
What This Paper Contributes to Our Knowledge
Transcutaneous measurements of CO2 and O2 did not accurately reflect arterial values in subjects with moderate to severe exacerbation of COPD. Agreement between changes in CO2 was acceptable.
Methods
This study was conducted at Bispebjerg Hospital from April to December 2019. An application to the regional ethics committee (H-19015292) was waived, and the study was approved by the hospital board of directors as a quality improvement study.
We included adult subjects (≥ 18 y old) who were either hospitalized acutely at a respiratory intermediate care unit or ICU for treatment of exacerbation of COPD with NIV or had recently been extubated after mechanical ventilation. Patients were excluded if inclusion was not possible within 24 h of initiation of NIV or within 2 h after extubation. Patients were also excluded if there were no arterial blood gas analyses scheduled in the 3 h following inclusion or if they had severe dermatological problems or known allergic reactions to the equipment.
We used the TCM5 Flex monitor connected to sensor 84 (Radiometer Medical, Brønshøj, Denmark). The sensor consists of a Stow-Severinghaus-type 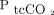 electrode combined with a Clark-type
electrode combined with a Clark-type  electrode. The sensor was automatically calibrated prior to every session, and the sensor membrane was changed as recommended by the manufacturer.
electrode. The sensor was automatically calibrated prior to every session, and the sensor membrane was changed as recommended by the manufacturer.
Transcutaneous sensors were placed simultaneously on both the anterior aspect of the subjects’ right upper arm and over the major pectoral muscle immediately after inclusion. The skin was inspected for suitability and then cleansed with 83% ethanol and allowed to dry before placement of an adhesive fixation ring. Two drops of contact gel were applied on the skin in the center of the fixation ring before positioning the sensor with light pressure for correct contact to skin surface. The sensor was automatically heated to 43.5°C, at which time monitoring began.
Stable values of  were reached after ∼ 10 min and for
were reached after ∼ 10 min and for  after an additional 10 min, presumably signifying full arterialization of the capillary bed. The attending health care staff was blinded to the transcutaneous measurements.
after an additional 10 min, presumably signifying full arterialization of the capillary bed. The attending health care staff was blinded to the transcutaneous measurements.
Routine ABG samplings from the radial artery were performed as part of standard clinical practice by either clinical ward staff or the investigator. The number and timing of ABGs were at the discretion of the attending physician. The time when blood filled the syringe was noted by the investigator as the time of ABG. The ABG samples were analyzed using a Radiometer ABL90 analyzer (Radiometer Medical).
Each subject was monitored for 3 h. Transcutaneous measurements of 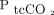 and
and  were stored with a sampling frequency of 1 measurement per second, and the values corresponding to time of ABG were calculated as time of ABG plus 2 min, recommended due to response and lag time of the sensor and monitoring system.15 We recorded sampling time and the results of up to 2 ABGs and the corresponding transcutaneous measurements during the monitoring period. Demographic data, clinical characteristics, physiological vital signs, and results of ABG analyses were obtained from the electronic medical records.
were stored with a sampling frequency of 1 measurement per second, and the values corresponding to time of ABG were calculated as time of ABG plus 2 min, recommended due to response and lag time of the sensor and monitoring system.15 We recorded sampling time and the results of up to 2 ABGs and the corresponding transcutaneous measurements during the monitoring period. Demographic data, clinical characteristics, physiological vital signs, and results of ABG analyses were obtained from the electronic medical records.
The primary outcome was agreement between the CO2 and O2 partial pressures of corresponding ABG and transcutaneous measurements (ie,  vs
vs 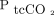 , and
, and  vs
vs  ). For the primary analysis we pooled concurrent measurements from sensors placed on the upper arm and the chest (ie, up to 2 measurements from each site) and compared both readings to results from the corresponding ABG analysis. We also compared agreement with ABGs between sensor placements. The secondary outcome was agreement between changes in the CO2 and O2 partial pressures of corresponding ABG and transcutaneous measurements from first to second sampling (ie,
). For the primary analysis we pooled concurrent measurements from sensors placed on the upper arm and the chest (ie, up to 2 measurements from each site) and compared both readings to results from the corresponding ABG analysis. We also compared agreement with ABGs between sensor placements. The secondary outcome was agreement between changes in the CO2 and O2 partial pressures of corresponding ABG and transcutaneous measurements from first to second sampling (ie, 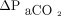 vs
vs  and
and 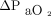 vs
vs 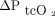 ). For this analysis, we compared transcutaneous readings from the arm and chest separately to corresponding ABG analyses.
). For this analysis, we compared transcutaneous readings from the arm and chest separately to corresponding ABG analyses.
Demographic and clinical characteristics were summarized in frequency tables using counts and percentages for categorical variables and means and standard deviation for continuous variables. Agreement between ABGs and transcutaneous measurements were determined by calculating the mean difference between the 2 methods and corresponding 95% CI for the 95% LoA, adding a random-effects model to the analysis, to account for multiple observations per individual.16 We used the method of variance estimates recovery to calculate the 95% CI for LoA.17 Results were plotted using standard Bland-Altman plots. Limits of agreement within ± 7.5 mm Hg were considered clinically acceptable.18 We compared agreement with ABGs of transcutaneous sensors placed on the arm and chest, respectively, using mixed linear models with difference between ABG and transcutaneous measurement as the response variable and sensor placement as the explanatory variable, with subject identication number as a random effect to account for multiple observations per individual. We analyzed the effect of the absolute level of 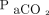 and
and 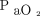 on agreement using mixed linear models with the absolute difference between ABGs and transcutaneous measurements as the response variable and
on agreement using mixed linear models with the absolute difference between ABGs and transcutaneous measurements as the response variable and 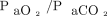 as the explanatory variable, with subject identication number as a random effect and adjusting for heteroscedasticity across blood gas levels. All data analyses were conducted using the statistical software R 3.6.2 and the rmcorr, lme4, and nlme add-on packages (R Foundation for Statistical Computing, Vienna, Austria).19-22
as the explanatory variable, with subject identication number as a random effect and adjusting for heteroscedasticity across blood gas levels. All data analyses were conducted using the statistical software R 3.6.2 and the rmcorr, lme4, and nlme add-on packages (R Foundation for Statistical Computing, Vienna, Austria).19-22
Results
Forty-three patients admitted with a confirmed diagnosis of exacerbation of COPD were screened, and 23 fulfilled an exclusion criterion (Fig. 1). The study included 20 subjects, of whom 16 subjects were monitored during NIV (median monitoring time: 2 h, 29 min) and 4 subjects were monitored shortly after extubation (median monitoring time: 2 h, 53 min). Ten subjects were male, mean ± SD age was 71.5 ± 8.7 y, and mean ± SD body mass index was 26.4 ± 7.7 kg/m2 (Table 1). Additional baseline characteristics of the subjects, such as respiratory values are presented in Table 1.
Flow chart. ABG = arterial blood gas analysis.
Subject Baseline Characteristics
We recorded 57 transcutaneous measurements (35 and 22 from sensors on the upper arm and chest, respectively) for comparison with concurrent ABGs at 36 time points (16 subjects had 2 ABGs analyzed during the monitoring period, and 4 subjects had only 1 ABG analyzed) (Fig. 1). In 29 of 36 ABGs,  was elevated above 45 mm Hg, signifying hypercapnia. Mean ± SD levels of transcutaneous and arterial blood gasses in the study population are presented in Table 2.
was elevated above 45 mm Hg, signifying hypercapnia. Mean ± SD levels of transcutaneous and arterial blood gasses in the study population are presented in Table 2.
Summary of ABG and Transcutaneous Measurements of CO2 and O2
The mean difference (bias) between 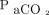 and
and  was 2.5 mm Hg with LoA of –10.6 to 15.6 mm Hg. The 95% CI for the lower LoA was –15.4 to –5.7 mm Hg; the 95% CI for the upper LoA was 10.8–17.1 mm Hg (Fig. 2). The mean difference between
was 2.5 mm Hg with LoA of –10.6 to 15.6 mm Hg. The 95% CI for the lower LoA was –15.4 to –5.7 mm Hg; the 95% CI for the upper LoA was 10.8–17.1 mm Hg (Fig. 2). The mean difference between 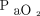 and
and 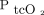 was 11.2 mm Hg with LoA of –28.2 to 50.6 mm Hg. The 95% CI for the lower LoA was –40.2 to –16.1 mm Hg; the 95% CI for the upper limit was 38.5–62.6 mm Hg (Table 3, Fig. 2).
was 11.2 mm Hg with LoA of –28.2 to 50.6 mm Hg. The 95% CI for the lower LoA was –40.2 to –16.1 mm Hg; the 95% CI for the upper limit was 38.5–62.6 mm Hg (Table 3, Fig. 2).
Bland-Altman plots of agreement between arterial blood gas analysis and transcutaneous measurements of 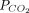 (A) and
(A) and  (B). Solid lines show mean bias; dotted lines show upper and lower 95% limits of agreement. Measurements from the same subject are connected by lines. The shaded area shows the predefined clinically acceptable limits of agreement of ± 7.5 mm Hg. Average measures were calculated as (arterial pressure + transcutaneous pressure)/2.
(B). Solid lines show mean bias; dotted lines show upper and lower 95% limits of agreement. Measurements from the same subject are connected by lines. The shaded area shows the predefined clinically acceptable limits of agreement of ± 7.5 mm Hg. Average measures were calculated as (arterial pressure + transcutaneous pressure)/2.
Bland-Altman Characteristics of Agreement Between ABG and Transcutaneous Measurements of CO2 and O2
The mean difference between agreement with ABGs of transcutaneous sensors placed on the upper arm and chest was 0.0 kPa (95% CI –0.01 to 0.08, P = .68) for CO2 and 0.1 kPa (95% CI –0.4 to 0.6, P = .77) for O2, as shown in Figure E1 (see the supplementary materials at http://www.rcjournal.com).
The O2 measurements of 2 subjects (no. = 8) appeared to be outliers (Fig. 2B) and were removed for sensitivity analyses. After removing these measurements, the mean difference between 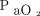 and
and 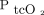 was 1.1 kPa with LoA of –1.9 to 0.64 kPa.
was 1.1 kPa with LoA of –1.9 to 0.64 kPa.
Linear regression analysis of the relationship between the absolute difference between 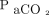 and
and  as a function of
as a function of 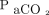 showed a slope of 0.07 (95% CI –0.04 to 0.17, P = .22), whereas the corresponding slope for O2 measurements was 0.48 (95% CI 0.21–0.76, P < .001).
showed a slope of 0.07 (95% CI –0.04 to 0.17, P = .22), whereas the corresponding slope for O2 measurements was 0.48 (95% CI 0.21–0.76, P < .001).
Mean ± SD time between 2 ABG measurements for the same subject was 95.1 ± 33 min. Mean changes in transcutaneous blood gasses, measured with the sensor placed on the arm and chest, and changes in arterial blood gasses between the 2 measurement time points are shown in Table E1 (see the supplementary materials at http://www.rcjournal.com).
Bland-Altman plots of paired changes in ABGs and transcutaneous measurements of CO2 and O2 are shown in Figure E3 (see the supplementary materials at http://www.rcjournal.com). The mean difference between  and
and 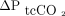 measured on the upper arm was 2.2 mm Hg with LoA of –4 to 8.3 mm Hg. The 95% CI of the lower limit was –6.6 to –1.3 mm Hg, and the 95% CI of the upper limit was 5.6–10.9 mm Hg; the mean difference between
measured on the upper arm was 2.2 mm Hg with LoA of –4 to 8.3 mm Hg. The 95% CI of the lower limit was –6.6 to –1.3 mm Hg, and the 95% CI of the upper limit was 5.6–10.9 mm Hg; the mean difference between  and
and  measured on the upper arm was –5.3 mm Hg with LoA of –37.9 to 27.4 mm Hg. The 95% CI of the lower limit was –52.1 to –23.6 mm Hg, and the 95% CI of the upper limit was 13.1–41.6 mm Hg (Table E2; see the supplementary materials at http://www.rcjournal.com). The mean difference between
measured on the upper arm was –5.3 mm Hg with LoA of –37.9 to 27.4 mm Hg. The 95% CI of the lower limit was –52.1 to –23.6 mm Hg, and the 95% CI of the upper limit was 13.1–41.6 mm Hg (Table E2; see the supplementary materials at http://www.rcjournal.com). The mean difference between  and
and  measured on the chest was 1.9 mm Hg with LoA of –2.6 to 6.5 mm Hg. The 95% CI for the lower limit was –5.3 to 0.1 mm Hg, and the 95% CI for the upper limit was 3.8–9.1 mm Hg; the mean difference between
measured on the chest was 1.9 mm Hg with LoA of –2.6 to 6.5 mm Hg. The 95% CI for the lower limit was –5.3 to 0.1 mm Hg, and the 95% CI for the upper limit was 3.8–9.1 mm Hg; the mean difference between  and
and  measured on the chest was –10 mm Hg with LoA –59.9 to 39.9 mm Hg. The 95% CI of the lower limit was –89.4 to –30.3 mm Hg, and the 95% CI for the upper limit was 10.3–69.4 mm Hg.
measured on the chest was –10 mm Hg with LoA –59.9 to 39.9 mm Hg. The 95% CI of the lower limit was –89.4 to –30.3 mm Hg, and the 95% CI for the upper limit was 10.3–69.4 mm Hg.
After removing outliers for O2 measurements (n = 2 from both upper arm and chest measurements), the mean difference between 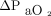 and
and 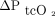 measured on the upper arm was 0.0 mm Hg with LoA of –15.8 to 16.5 mm Hg. The mean difference between
measured on the upper arm was 0.0 mm Hg with LoA of –15.8 to 16.5 mm Hg. The mean difference between 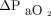 and
and  measured on the chest was 1.5 mm Hg with LoA of –21.0 to 24.0 mm Hg.
measured on the chest was 1.5 mm Hg with LoA of –21.0 to 24.0 mm Hg.
Discussion
Transcutaneous measurements of CO2 and O2 underestimated ABG values by 2.5 and 11.2 mm Hg, respectively, in this observational study of subjects with an exacerbation of COPD. Limits of agreement were wide, and all exceeded the predefined clinically acceptable threshold of ± 7.5 mm Hg. Changes in CO2 detected with transcutaneous measurements were within acceptable limits of agreement with ABGs, although the 95% CI around the limits of agreement were wide.
Previous reports on the accuracy of transcutaneous monitoring of blood gases have been conflicting. In a study of 40 elderly subjects admitted due to acute cardiac or pulmonary disorders, Janssens et al23 reported that  only underestimated
only underestimated 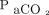 by 0.075 mm Hg with 95% LoA of –8.3 to 8.3 mm Hg, making it compatible with clinical use, while the reported bias for
by 0.075 mm Hg with 95% LoA of –8.3 to 8.3 mm Hg, making it compatible with clinical use, while the reported bias for 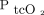 was large and comparable to our findings. Similarly, in a study of 25 subjects with asthma or pneumonia, Perrin et al24 reported a bias for
was large and comparable to our findings. Similarly, in a study of 25 subjects with asthma or pneumonia, Perrin et al24 reported a bias for 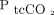 of –0.075 mm Hg with 95% LoA of –3.8 to 3.8 mm Hg.
of –0.075 mm Hg with 95% LoA of –3.8 to 3.8 mm Hg.
In contrast, several recent studies14,15,25 of hypercapnic subjects in acute respiratory distress have reported that 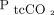 could significantly underestimate
could significantly underestimate 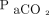 , which is also suggested by our findings. Some of these studies reported decreasing reliability of
, which is also suggested by our findings. Some of these studies reported decreasing reliability of 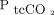 with increasing levels of hypercapnia.14,25 In our study, accuracy of
with increasing levels of hypercapnia.14,25 In our study, accuracy of 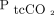 appeared to be stable across the mainly hypercapnic range of
appeared to be stable across the mainly hypercapnic range of 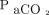 .
.
Removing outliers at the high range of  improved agreement for O2 measurements, but linear regression analysis adjusted for the apparent heterogeneity of variance suggests that
improved agreement for O2 measurements, but linear regression analysis adjusted for the apparent heterogeneity of variance suggests that 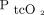 measurements are more erratic at higher levels of
measurements are more erratic at higher levels of 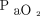 (eg, patients receiving oxygen supplementation). The reasons for the mentioned discrepancies are uncertain but may include differences in patient populations in terms of disease process and severity; make of monitoring equipment; differences in staff training regarding device use; or unknown effects of clinical signs of respiratory failure such as altered skin perfusion, sweating, and fever.
(eg, patients receiving oxygen supplementation). The reasons for the mentioned discrepancies are uncertain but may include differences in patient populations in terms of disease process and severity; make of monitoring equipment; differences in staff training regarding device use; or unknown effects of clinical signs of respiratory failure such as altered skin perfusion, sweating, and fever.
A major strength of this study is the close monitoring of subjects hospitalized with exacerbation of COPD, the majority being in a hypercapnic state, allowing for evaluation of the accuracy of transcutaneous monitoring of blood gases in a highly relevant clinical setting. The study also included repeated measurements per subject, increasing statistical power and allowing for analysis of agreement in detection of changes in blood gases over time, which may be equally important in clinical practice.
Some limitations must be noted. First, ABGs were only drawn upon clinical indication, and we only recorded 1 measurement for a subset of subjects. We accounted for this unbalanced data structure in the statistical analyses. Second, a variety of areas for sensor placement is suggested by the manufacturer. In a large meta-analysis of 73 studies, including 7,021 paired measurements of transcutaneous and arterial 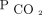 , Conway et al11 concluded that sensors should preferentially be applied to the earlobe and that users should consider setting the temperature of the sensor higher than 42°C when monitoring at other sites. For this study, the upper arm and the area over the major pectoralis muscle were chosen to avoid entanglement with the NIV mask and a sensor temperature of 43.5°C was used. Some transcutaneous measurements from the chest were not recorded due to technical issues at this sensor placement, and a relatively larger part of the total number of transcutaneous measurements (ie, 35 vs 22) were derived from the sensors placed on the upper arm. Moreover, the smaller number of transcutaneous measurements at the chest may have hampered our ability to analyze agreement with ABG analysis in detection of changes in blood gases for this sensor placement. However, we did not find any difference in agreement with ABGs between the 2 sensor placements, and we believe that both pooled observations and observations from separate sensor placements accurately reflect the performance of the transcutaneous measurements. Third, the time lag from initiation of monitoring with the TCM5 Flex until reliable values of blood gases were obtained (ie, 10 min for
, Conway et al11 concluded that sensors should preferentially be applied to the earlobe and that users should consider setting the temperature of the sensor higher than 42°C when monitoring at other sites. For this study, the upper arm and the area over the major pectoralis muscle were chosen to avoid entanglement with the NIV mask and a sensor temperature of 43.5°C was used. Some transcutaneous measurements from the chest were not recorded due to technical issues at this sensor placement, and a relatively larger part of the total number of transcutaneous measurements (ie, 35 vs 22) were derived from the sensors placed on the upper arm. Moreover, the smaller number of transcutaneous measurements at the chest may have hampered our ability to analyze agreement with ABG analysis in detection of changes in blood gases for this sensor placement. However, we did not find any difference in agreement with ABGs between the 2 sensor placements, and we believe that both pooled observations and observations from separate sensor placements accurately reflect the performance of the transcutaneous measurements. Third, the time lag from initiation of monitoring with the TCM5 Flex until reliable values of blood gases were obtained (ie, 10 min for 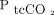 and 20 min for
and 20 min for  ) was a challenge in cases where sensor replacement was indicated, either because of unintended disconnection or periodic repositioning. Furthermore, on 2 occasions the system performed a mandatory stabilization immediately after startup without any previous warning. This process takes from 40 min to 8 h, according to the user guide provided by the manufacturer, and resulted in 2 subjects only being monitored with sensors on the upper arm in this study. More importantly, such delays may severely hamper use of the system for monitoring critically ill patients in a clinical setting.
) was a challenge in cases where sensor replacement was indicated, either because of unintended disconnection or periodic repositioning. Furthermore, on 2 occasions the system performed a mandatory stabilization immediately after startup without any previous warning. This process takes from 40 min to 8 h, according to the user guide provided by the manufacturer, and resulted in 2 subjects only being monitored with sensors on the upper arm in this study. More importantly, such delays may severely hamper use of the system for monitoring critically ill patients in a clinical setting.
In this study, there was a clinically acceptable agreement between changes in ABG values and transcutaneous measurements 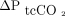 , as previously observed,15 but not for
, as previously observed,15 but not for 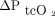 . This is clinically relevant because changes to
. This is clinically relevant because changes to 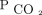 serve as an important marker of treatment response in patients with exacerbation of COPD including during NIV. Transcutaneous measurements allow for continuous monitoring of changes in
serve as an important marker of treatment response in patients with exacerbation of COPD including during NIV. Transcutaneous measurements allow for continuous monitoring of changes in  and may reduce the need for repeated arterial punctures, thus improving patient comfort.6 However, the wide LoA ranges for single-point estimates reported in this study, which allow for substantial underestimation (ie, up to > 15 mm Hg) of absolute values of
and may reduce the need for repeated arterial punctures, thus improving patient comfort.6 However, the wide LoA ranges for single-point estimates reported in this study, which allow for substantial underestimation (ie, up to > 15 mm Hg) of absolute values of 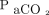 , present a safety concern, as noted by Kelly and Klim.25 A recent review proposed negating this problem by drawing a reference ABG at the start of transcutaneous monitoring to detect a possible gap between
, present a safety concern, as noted by Kelly and Klim.25 A recent review proposed negating this problem by drawing a reference ABG at the start of transcutaneous monitoring to detect a possible gap between 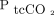 and
and  and then calibrating equipment accordingly.26
and then calibrating equipment accordingly.26
In this study, transcutaneous monitoring of 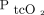 was in poor agreement with
was in poor agreement with 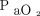 in terms of both point estimates and changes.
in terms of both point estimates and changes. 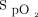 is a pseudo-marker for
is a pseudo-marker for 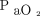 , so oxygenation status can already be continuously monitored using pulse oximetry.27 Hence the need for continuous monitoring of
, so oxygenation status can already be continuously monitored using pulse oximetry.27 Hence the need for continuous monitoring of  may be limited, while monitoring of
may be limited, while monitoring of 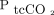 provides additional valuable information to clinicians in an ICU setting as well as in general medical wards.
provides additional valuable information to clinicians in an ICU setting as well as in general medical wards.
Conclusions
Transcutaneous measurements of CO2 and O2 did not accurately reflect arterial values in subjects with moderate to severe exacerbation of COPD. Agreement between changes in CO2 was acceptable, and transcutaneous monitoring may be used for continuous monitoring of 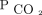 in conjunction with ABG analysis for reference.
in conjunction with ABG analysis for reference.
Footnotes
- Correspondence: Christian S Meyhoff MD PhD, Bispebjerg Bakke 23, 2400 Copenhagen, NV, Denmark. E-mail: christian.sylvest.meyhoff{at}regionh.dk
Mr Sørensen and Dr Leicht are co-first authors.
Drs Aasvang and Meyhoff are joint senior authors.
Supplementary material related to this paper is available at http://www.rcjournal.com.
This work was supported in part by the Innovation Fund Denmark (8056-00055B); the Danish Cancer Society (R150-A9865-16-S48); Copenhagen Center for Health Technology (CACHET); Radiometer; A.P. Møller Foundation; Bispebjerg and Frederiksberg Hospital; Rigshospitalet; and the Technical University of Denmark. The WARD-project has received grants from the Innovation Fund Denmark, the Novo Nordic Foundation, the Danish Cancer Society, Steno Diabetes Center Denmark, Copenhagen Center for Health Technology, Radiometer, A.P. Møller Foundation as well as internal institutional funding. Drs Meyhoff and Aasvang are co-founders of a start-up company, WARD247 ApS, with the aim of pursuing the regulatory and commercial activities of the WARD-project. WARD247 ApS has finalized terms for license agreement for any WARD-project software and patents. There are currently no patents pending or filed. None of the above entities have influence on the study design, conduct, analysis or reporting.
Dr Meyhoff discloses relationships with Merck Sharp & Dohme, Boehringer Ingelheim, and Radiometer. Dr Aasvang discloses relationships with Norpharma A/S and Radiometer. Dr Elvekjaer discloses a relationship with Merck Sharp & Dohme. The remaining authors have no conflicts to disclose.
- Copyright © 2021 by Daedalus Enterprises









