Abstract
Endotracheal intubation for airway management is a common procedure in the ICU. Intubation may be difficult due to anatomic airway abnormalities but also due to physiologic derangements that predispose patients to cardiovascular collapse during the procedure. Results of studies demonstrate a high incidence of morbidity and mortality associated with airway management in the ICU. To reduce the likelihood of complications, medical teams must be well versed in the general principles of intubation and be prepared to manage physiologic derangements while securing the airway. In this review, we present relevant literature on the approach to endotracheal intubation in the ICU and provide pragmatic recommendations relevant to medical teams performing intubations in patients who are physiologically unstable.
Introduction
Endotracheal intubation is the third-most frequently performed medical procedure in United States hospitals.1 Airway management of patients who are critically ill can present anatomic, physiologic, and logistic challenges. Within the ICU, intubation is associated with high complication rates, up to 40% in some series,2 with hypoxemia and hypotension being among the most common and cardiac arrest being the most severe.3 The rising prevalence of obesity,4 which is known to be associated with a higher risk of peri-intubation complications,5 further increases the risk of intubations performed in the ICU. The physiologically difficult airway is defined by the presence of physiologic derangements, which increase the risk of cardiovascular collapse during intubation, independent of the anatomic difficulty.6 This review summarizes the literature on the physiologically difficult airway, as frequently performed by medical teams in the ICU, and provides recommendations on potential approaches to intubation under various clinical circumstances.
Preparing for Intubation in the ICU
Intubation checklists should be considered in the ICU setting to prepare the team and relieve the cognitive burden of the procedure. The Difficult Airway Society has proposed such a checklist to which readers may refer.7 Although a recent meta-analysis found no reduction in mortality associated with intubation checklists, their use may reduce peri-intubation hypoxemia.8
Assessing for Airway Difficulty
Anticipation of a difficult intubation is critical, and the intubation history, if known, should be reviewed. Vital signs, body habitus, airway anatomy, laboratory studies, co-morbidities, and indication for intubation all influence the approach. In the ICU, the only validated prediction tool for difficult intubation is the MACOCHA score (Table 1).9 A score of ≥3 predicts difficult intubation, with 73% sensitivity and 89% specificity; scores < 3 have a negative predictive value of 98%. However, it is not always feasible to obtain a complete MACOCHA score (eg, Mallampati score) in patients who are critically ill. Such scenarios should be approached with the anticipation for a difficult airway.
MACOCHA Score
Assembling the Team
Clearly assigning roles before intubation facilitates teamwork and communication. Intubation team roles and composition have been proposed by the Difficult Airway Society and include the following: primary intubator, secondary intubator, drug administrator, vital sign monitor, and runner (to obtain additional supplies or support if needed), among others.7 Some members, depending on the team size, may serve multiple roles. ICU teams should adopt the composition that best fits their clinical practice.
Preparing the Equipment
Vital sign monitors should be clearly visible and have audible alarm limits set for pulse oximetry and blood pressure when feasible. Intubation equipment should include endotracheal tubes (of various sizes), direct laryngoscopes, video laryngoscope, supraglottic airway, stylet, tracheal tube introducer, suction, oxygen face mask, carbon dioxide detector and capnography, and ventilator. In addition, an airway supply cart should be readily available for every ICU intubation.10 A bronchoscope may be available, on standby, outside the room. All medications should be prepared for administration (Table 2).
Common Medications for the Physiologically Difficult Airway in the ICU
Formulating the Plan
The most important objective of the intubation plan during a physiologically difficult airway is in maximizing the first-attempt success because multiple attempts have been associated with an increased risk of complications.11,12 One study of emergency intubations outside the operating room reported the incidence of hypoxemia increased from 10% to 70% and cardiac arrest increased from 0.7% to 11% when > 2 intubation attempts were required.13
Techniques that have improved first-attempt success include rapid-sequence induction and administration of neuromuscular blocking agents.14,15 As such, guidelines of multiple societies recommend use of rapid-sequence induction and neuromuscular blocking agents for intubation of patients who are critically ill.7,16,17 The superiority of video laryngoscopy compared with direct laryngoscopy on first-attempt success remains controversial. A 2017 Cochrane review found moderate evidence that video laryngoscopy reduced failed intubations in subjects with anticipated difficult airways compared with direct laryngoscopy and improved the glottic view; however, there was no difference in first-attempt success, incidence of hypoxemia, or mortality.18 Airway societies recommend that video laryngoscopy be available for all ICU intubations and considered from the outside if a difficult intubation is predicted.7,16
Intubation with an endotracheal tube and stylet has been shown to improve first-attempt success in the ICU compared with an endotracheal tube without a stylet.19 Among subjects with at least one difficult airway feature in the emergency department, use of a tracheal tube introducer, also known as a “bougie,” resulted in greater first-attempt success compared with an endotracheal tube with a stylet in one randomized trial.20 However, a recent multi-center randomized trial found no difference in first-attempt success with a bougie compared with a stylet among ICU and emergency department intubations, the majority of which were performed by using video laryngoscopy.21 Use of a stylet or bougie is recommended for intubation of patients who are critically ill, and the decision of which to use may depend on individual preference and experience.
The intubation plan should be clear to the entire team before performing the procedure. The Difficult Airway Society recommends a “Plan A-B-C” approach to intubation of adults who are critically ill.7 Plan A entails administration of medications and tracheal intubation via laryngoscopy. Plans B and C comprise the “rescue airway” scenario, during which supraglottic airway insertion is attempted and bag-mask ventilation is continued between attempts. Advances in supraglottic airways are reviewed elsewhere.22 To reduce the cognitive burden in an airway emergency, the “Vortex” approach has been proposed (but not yet supported by evidence).23 The Vortex approach is based on the 3 non-surgical techniques for establishing a patent airway: face-mask ventilation, supraglottic airway insertion, and endotracheal intubation. Any technique may be attempted, but no more than 3 times (one attempt by the most experienced clinician) before proceeding to the next technique. If best efforts at all 3 techniques are unsuccessful or there is clinical deterioration of the patient, then the team must declare a “cannot intubate, cannot ventilate” situation and proceed with front-of-neck airway.23,24 Front-of-neck airway insertion has been previously reviewed.7,24–26
Preparing the Patient
Reliable intravenous access should be obtained before intubation. The patient should also be positioned appropriately for preoxygenation and intubation. Intubation of the patient is preceded by preoxygenation whenever feasible. Preoxygenation refers to delivery of a high 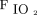 before administration of induction agents and neuromuscular blocking agents, which result in denitrogenation of the functional residual capacity (FRC) and creation of an oxygen-rich reservoir.27 Preoxygenation facilitates longer apneic time before onset of critical hypoxemia.28 Preoxygenation is typically performed via high-flow nasal cannula (HFNC), noninvasive ventilation (NIV), or bag-mask ventilation with PEEP. Airway society guidelines recommend at least 3 min of preoxygenation or 8 vital capacity breaths.29 Extending the preoxygenation time beyond 3 min does not necessarily further reduce the risk of oxygen desaturation because 25% of subjects in one study had worsening hypoxemia with prolonged preoxygenation (8 min).30 Gas exchange impairment (eg, a shunt) may limit the highest achievable
before administration of induction agents and neuromuscular blocking agents, which result in denitrogenation of the functional residual capacity (FRC) and creation of an oxygen-rich reservoir.27 Preoxygenation facilitates longer apneic time before onset of critical hypoxemia.28 Preoxygenation is typically performed via high-flow nasal cannula (HFNC), noninvasive ventilation (NIV), or bag-mask ventilation with PEEP. Airway society guidelines recommend at least 3 min of preoxygenation or 8 vital capacity breaths.29 Extending the preoxygenation time beyond 3 min does not necessarily further reduce the risk of oxygen desaturation because 25% of subjects in one study had worsening hypoxemia with prolonged preoxygenation (8 min).30 Gas exchange impairment (eg, a shunt) may limit the highest achievable 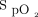 during preoxygenation in patients who are hypoxemic. Preoxygenation in an upright position is recommended to maximize the FRC.3
during preoxygenation in patients who are hypoxemic. Preoxygenation in an upright position is recommended to maximize the FRC.3
During intubation, the patient is positioned in a manner that optimizes the alignment of the oral, pharyngeal, and laryngeal axes, which allows visualization of the vocal cords. The two most common patient positions for intubation are “sniffing” and “ramped.”31–33 In the sniffing position, the patient is supine (with the bed flat or elevated) with the neck flexed forward and the head extended. In the ramped position, the patient's torso and head are elevated, typically 20° to 30°, and the neck is extended so that the earlobe and sternum are in parallel.34–36 The ramped position increases FRC when used in the operating room for elective intubations, which reduces the risk of hypoxemia.34–36 A multi-ICU trial that compared sniffing versus the ramped position found no difference in the lowest median peripheral oxygen saturation but found that the ramped position was associated with worse Cormack-Lehane grade views and a lower first-attempt intubation success.37 One caveat is that the majority of the subjects in the trial were not obese and the ramped position may cause misalignment of the axes via overflexion of the neck in patients who are not obese. Ramped is the preferred position for patients who are obese.38
Physiologically Difficult Airway Scenarios
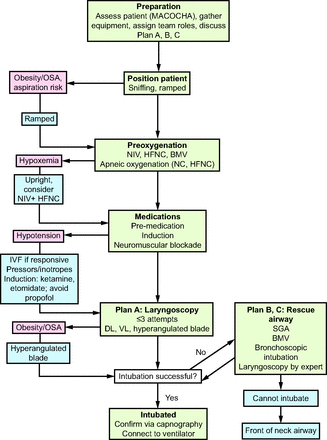
In each of the following scenarios, the patient is at increased risk of cardiovascular collapse during intubation. The goal of the team should be to support the cardiovascular and pulmonary systems and maximize first-attempt success to mitigate complications. See Table 3 for key recommendations and Figure 1 for a physiologically difficult airway algorithm.
Physiologically difficult airway algorithm. OSA = obstructive sleep apnea; NIV = noninvasive ventilation; HFNC = high-flow nasal cannula; BMV = bag-mask ventilation; IVF = intravenous fluid; DL = direct laryngoscopy; VL = video laryngoscopy; SGA = suppraglottic airway.
Recommendations for Respiratory and Hemodynamic Support During Physiologically Difficult Airway Scenarios
Hypoxemic Respiratory Failure
Hypoxemia occurs in ∼20% of intubations performed among patients who are critically ill.3 Acute hypoxemic respiratory failure and low peripheral oxygen saturation at induction are the strongest predictors of severe hypoxemia during intubation.39 Studies that investigated the optimal delivery method of preoxygenation in subjects who were hypoxemic show varied results.40–43 In a meta-analysis of 7 randomized controlled trials that included 959 subjects, NIV was associated with fewer episodes of severe hypoxemia (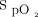 < 80%) compared with HFNC or low-flow nasal cannula.40 A randomized trial found no difference in severe hypoxemia with HFNC compared with NIV.41 However, the subgroup of subjects with
< 80%) compared with HFNC or low-flow nasal cannula.40 A randomized trial found no difference in severe hypoxemia with HFNC compared with NIV.41 However, the subgroup of subjects with 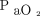 /
/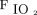 < 200 mm Hg before intubation had fewer hypoxemic events with NIV. As such, NIV may be the preferred modality in patients with more severe hypoxemia and in those with a reduced FRC and increased shunt fraction (eg, ARDS). HFNC is a reasonable alternative to NIV and may be preferable in patients with less severe hypoxemia. In a randomized trial, HFNC was not associated with lower nadir
< 200 mm Hg before intubation had fewer hypoxemic events with NIV. As such, NIV may be the preferred modality in patients with more severe hypoxemia and in those with a reduced FRC and increased shunt fraction (eg, ARDS). HFNC is a reasonable alternative to NIV and may be preferable in patients with less severe hypoxemia. In a randomized trial, HFNC was not associated with lower nadir 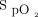 and had fewer adverse events compared with bag-mask ventilation in subjects with non-severe hypoxemia.43 Another trial found the combination of HFNC and NIV to be superior to NIV alone42; however, it may be difficult logistically to administer both simultaneously.29
and had fewer adverse events compared with bag-mask ventilation in subjects with non-severe hypoxemia.43 Another trial found the combination of HFNC and NIV to be superior to NIV alone42; however, it may be difficult logistically to administer both simultaneously.29
Efforts to minimize hypoxemia after induction of anesthesia and neuromuscular blockade are especially important in patients with hypoxemic respiratory failure. Apneic oxygenation refers to passive oxygenation during intubation while the patient is no longer breathing. Airway society guidelines recommend apneic oxygenation with low-flow nasal cannula at 15 L/min or HFNC.7,29 These devices produce high oxygen concentrations in the hypopharynx, which then allows passive replacement oxygen consumed from the FRC. Bag-mask ventilation use between induction and laryngoscopy has also been associated with higher 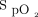 nadir and less-severe hypoxemia compared with no ventilation, and there has been no increase in reported aspiration events with its use.44 Although apneic oxygenation and bag-mask ventilation may provide some benefit, adequate preoxygenation is of greater importance.29
nadir and less-severe hypoxemia compared with no ventilation, and there has been no increase in reported aspiration events with its use.44 Although apneic oxygenation and bag-mask ventilation may provide some benefit, adequate preoxygenation is of greater importance.29
Rapid-sequence induction, followed by laryngoscopy, is the recommended method of induction and intubation in most patients.7,16,17 In cases of severe refractory hypoxemia, awake tracheal intubation can also be considered.3,45 During awake tracheal intubation, a patient who is awake and spontaneously breathing is intubated via bronchoscopy. The airway is first anesthetized by using lidocaine, which can be administered via nebulization, spray, transtracheal injection, gargle, or combinations thereof (not to exceed total 9 mg/kg).45 Sedation is often required, and agents with minimum respiratory depressant effects, such as dexmedetomidine or low-dose ketamine, are preferred. After adequate topical anesthesia and sedation, the bronchoscope (loaded with an endotracheal tube) is introduced into the airway. Additional lidocaine is instilled to ensure that the larynx is fully anesthetized. The bronchoscope is then advanced into the trachea until the carina is visualized. Finally, the endotracheal tube is advanced over the bronchoscope and secured in position.
Hypotension and Shock
Between 7.7% and 43% of intubations performed in the ICU are complicated by hypotension or cardiovascular instability.3,46 Furthermore, the incidence of cardiac arrest associated with intubation is ∼2.7% among the patients who are critically ill.47 Patients who experience peri-intubation cardiovascular instability have an increased risk of ICU mortality, with an odds ratio of 2.47.48 A shock index (heart rate/systolic blood pressure) of ≥0.8 before rapid-sequence induction is a strong predictor of patients at risk for post-intubation hypotension.49 Accurate hemodynamic monitoring is essential in these patients, and placement of an intra-arterial catheter is preferred if feasible.
Hemodynamic optimization through rapid infusion of fluids and vasopressors is hypothesized to prevent severe complications,7,29 although empiric administration of a 500-mL fluid bolus did not reduce cardiovascular collapse or mortality in a randomized trial.50 However, current airway society recommendations are for patients who are fluid responsive to receive fluid administration before or during intubation.29 Patients not responding to fluids should be started on a vasopressor infusion with norepinephrine, epinephrine, or phenylephrine.29 Norepinephrine and epinephrine are favored in the setting of myocardial suppression due to their inotropic properties. Vasopressor boluses (phenylephrine or epinephrine) should be used if there will be a delay in starting a continuous infusion.
An analysis of the International Observational Study to Understand the Impact and Best Practices of Airway Management in Critically Ill Patients (INTUBE) study identified induction with propofol to be a modifiable risk factor associated with increased risk of peri-intubation cardiovascular instability.48 As such, propofol should generally be avoided for induction in patients who are critically ill and with hemodynamic compromise. Etomidate and ketamine are the recommended “hemodynamically neutral” agents in this population.29 Etomidate has been demonstrated to cause adrenal insufficiency and increased steroid requirement in patients who are in septic shock.51,52 However, this effect typically reverses after 48 h.51 A meta-analysis of 5,552 subjects found that etomidate for rapid-sequence induction was not associated with increased mortality in septic shock.52 Ketamine is often selected for its sympathomimetic properties; however, it can cause hypotension or myocardial depression in patients with catecholamine-depleted states.29,53 A recent randomized trial found greater survival at 7 d after intubation with ketamine compared with etomidate; however, there was no difference in survival at day 28.54 One caveat of this study is that the majority of the subjects were not in shock at the time of intubation. Overall, both etomidate and ketamine are reasonable choices in patients who are hypotensive, and their potential benefits and adverse effects should be weighed on a case-by-case basis (eg, cautious use of etomidate in sepsis). Lastly, if the etiology of shock is known and readily addressable (eg, needle decompression for tension pneumothorax), this should be performed before intubation whenever possible.
Obesity
Among a cohort of 1,400 subjects intubated while in the ICU, 20% were obese.5 Compared with an operating room cohort, these subjects had twice the risk of having a difficult intubation.5 Furthermore, the risk of severe life-threatening complications of intubation was 20 times greater for subjects who were obese and in the ICU than in the operating room.5 Elevated Mallampati scores and frequent concomitant obstructive sleep apnea among patients who were obese predict difficult intubation.5,9 Several factors further increase the difficulty of intubating patients who are obese. Excess facial and neck adiposity impairs face-mask ventilation and makes anatomic landmarks difficult to identify.55 Increased chest wall mass and upward displacement of the diaphragm from elevated intra-abdominal pressure may reduce the FRC and promote atelectasis, which results in ventilation/perfusion mismatch and oxygen desaturation. This is exacerbated when patients are supine, anesthetized, and receiving neuromuscular blockade.55
The safe apneic time is reduced in obesity, which makes efficiency critical. The ramped position is preferred because it may minimize the reduction in FRC.38,56 Preoxygenation with NIV has been shown to improve oxygenation and prolong safe apneic time in patients who are morbidly obese.57,58 A post hoc analysis of a randomized trial that compared preoxygenation with HFNC with NIV found no difference between them in degree or frequency of hypoxemia among subjects who were obese.59 Although NIV is generally preferred,7,56 the possible role of HFNC warrants further study. Video laryngoscopy is the recommended approach in patients who are obese.7,56 A randomized trial found better glottic views and less difficulty with a hyperangulated video laryngoscopy blade compared with direct laryngoscopy for subjects who were obese.60
Pulmonary Hypertension and Right-Ventricular Failure
Intubation in the setting of decompensated pulmonary hypertension and right-ventricular failure can precipitate hemodynamic collapse and cardiac arrest.29 If possible, patients with known pulmonary hypertension or concern for acute right heart failure (eg, in the setting of acute massive pulmonary embolism) should be assessed for severe right-ventricular dysfunction before intubation with transthoracic echocardiography. Bedside assessment of volume status with ultrasonography may be helpful to guide fluid management. Patients may require small intravenous fluid boluses or, particularly in this population, diuresis peri-intubation, depending on right-ventricular preload. Vasopressors with inotropic effects, such as epinephrine, or those with no effect on the pulmonary vasculature, such as vasopressin, should be administered early to augment the mean arterial pressure. It is recommended that blood pressure be monitored continuously with an intra-arterial catheter,61 with a goal to avoid sudden shifts in blood pressure around the time of intubation.
Hypoxemia and acidemia should also be corrected peri-intubation to mitigate further decompensation. Inhaled pulmonary vasodilators are recommended to reduce right-ventricular afterload and should ideally be started as part of overall pulmonary hypertension optimization but should not delay intubation if emergent.29,61 The upright position may be preferable due to increased right-ventricular preload and potential for increased distention while supine.61 HFNC can be used for preoxygenation and apneic oxygenation as well as administration of inhaled pulmonary vasodilators. Etomidate and ketamine are the preferred induction agents. Ultimately, intubation should be avoided in decompensated pulmonary hypertension or right-ventricular failure if possible. Pre-intubation extracorporeal membrane oxygenation should be considered in appropriate patients who may clearly be bridged to recovery or have an established path to bridge to transplantation in centers with extracorporeal membrane oxygenation capability.29,61,62
Large Pericardial Effusion or Tamponade
Intubation of patients with large pericardial effusions or tamponade can quickly lead to cardiac arrest given the effects of positive-pressure ventilation and the agents used to sedate the patient.63,64 Bedside echocardiography can detect a pericardial effusion and signs of impending tamponade and should be performed when possible. Peri-intubation strategies to prevent cardiovascular collapse include intravenous fluid administration to maintain preload, avoiding bradycardia and dysrhythmias to preserve cardiac output and ventricular filling, and administration of inotropes and pressors to maintain myocardial contractility and systemic vascular resistance. Ketamine and etomidate are the preferred induction agents given their lesser myocardial suppression effects. Positive-pressure ventilation may significantly reduce venous return and lead to acute decompensation, and, therefore, should be minimized. In cases of hemodynamic instability, pericardiocentesis before intubation may be required to prevent cardiovascular collapse.63 Surgery or interventional cardiology teams should be on standby for possible emergent pericardiocentesis.
Metabolic Acidosis
Intubation of patients with severe metabolic acidosis is complicated due to loss of the patient's respiratory compensation during the procedure and the need to maintain minute ventilation after intubation. Preoxygenation with NIV, if readily available, may decrease work of breathing and help estimate the ventilatory requirement.29 There is little consensus for dosing with sodium bicarbonate to mitigate acidosis during intubation. Attempting to match minute ventilation after intubation is very important, particularly if sedation, induction, or neuromuscular blockade was administered during intubation.65 Pressure support modes of ventilation may be useful once the patient resumes spontaneous breathing.
Status Asthmaticus
Intubation should be avoided in asthma exacerbations if possible but may be required in critical asthma.66 NIV can be considered for preoxygenation to assist ventilation peri-intubation.67 Ketamine and propofol are attractive options for rapid-sequence induction because they induce bronchodilation and, therefore, may reduce airway resistance both during and after intubation.66 After intubation, it is important to limit the set tidal volume and breathing frequency to allow adequate expiratory time to avoid breath-stacking and auto-PEEP.67 This may entail tolerating permissive hypercapnia (eg, pH 7.15–7.20) until airway resistance from bronchospasm, inflammation, and edema improves. Patients with refractory status asthmaticus after endotracheal intubation despite ventilator optimization should be considered early for extracorporeal carbon dioxide removal.68
Pregnancy
Obstetric ICU admissions in the United States range from 0.5 to 4 per 1,000 deliveries.69 Although acute respiratory failure occurs in < 0.1% of pregnancies, it is a common cause for obstetric admission to the ICU.70 Several physiologic changes in pregnancy complicate airway management. Reduced FRC and increased basal metabolic rate shorten the safe apneic time.69 Oxygen requirements of the mother and fetus are greater than the nonpregnant population, which further increases the importance of preoxygenation.70 Decreased lower esophageal sphincter tone and increased intra-abdominal pressure raise aspiration risk.69 Rapid-sequence induction is recommended due to an elevated aspiration risk.70 All obstetric intubations should be anticipated as difficult. The most experienced intubator available should perform the intubation.
High Aspiration Risk
Aspiration was historically one of the most feared complications of intubation. Although it now occurs in < 6% of ICU intubations,3 aspiration is associated with considerable morbidity and mortality. Patients with food in their stomachs; bowel obstruction; gastroparesis; and those with active emesis, hematemesis, or hemoptysis are at high risk for aspiration. In patients with high gastric volumes, decompression with a nasogastric tube is recommended, but data for this are lacking.3,7 It is recommended that patients at risk for aspiration be in the ramped position for preoxygenation and laryngoscopy.3
Rapid-sequence induction was initially developed as a technique to reduce the risk of aspiration.71 Adequate suction is critical when the oropharynx is contaminated with blood or vomitus because the debris can impair visualization. Application of cricoid pressure (Sellick maneuver) is often used during intubation to reduce aspiration; however, it remains controversial. A large randomized trial found no difference in aspiration rates for subjects who received cricoid pressure compared with sham, and its use was associated with higher rates of Cormack-Lehane grade 3 and 4 views and longer intubation time.72 There is insufficient evidence to support the routine use of cricoid pressure. Direct laryngoscopy is often preferred in the setting of upper gastrointestinal bleeding because blood can obscure the camera in video laryngoscopy; however, a retrospective analysis of > 300 emergency department intubations for gastrointestinal bleeding found no difference in first-pass success or laryngeal view grade between direct laryngoscopy and video laryngoscopy.73
COVID-19
The COVID-19 pandemic has resulted in > 675 million infections and 6.8 million deaths worldwide according to the Johns Hopkins Coronavirus Resource Center (https://coronavirus.jhu.edu/map.html. Accessed February 26, 2023). Endotracheal intubation is required in the most-severe cases, and hypoxemia and hypotension are common complications in these patients.74 In addition to the management of challenging physiology, the safety of the medical team must be prioritized due to the highly infectious nature of the virus. Consensus guidelines for safety protocols are available.75 Key principles include limiting the staff present at intubation, wearing full personal protective equipment, using negative-pressure rooms when possible, and maximizing the first-attempt success. The most experienced intubator should perform the procedure. Preoxygenation with a tight-fitting face mask is preferred to HFNC, NIV, or bag-mask ventilation due to aerosolization risk.75,76 Rapid-sequence induction has been associated with first-pass success.77 Video laryngoscopy is recommended over direct laryngoscopy.74,75,79
Summary
Intubation in the ICU presents challenges beyond difficult airway anatomy. Patients who are critically ill are generally more physiologically unstable than are patients who are intubated in operating room settings, with a greater risk for cardiovascular and pulmonary decompensation. The increased prevalence of obesity among patients who are critically ill further complicates routine intubation in this setting. Successful intubation of patients in the ICU requires that medical teams manage physiologically difficult scenarios and approach the procedure in such a way as to maximize first-attempt success.
Footnotes
- Correspondence: Max R O'Donnell MD, Division of Pulmonary, Allergy, and Critical Care Medicine, Columbia University Irving Medical Center, 622 West 168th Street, PH-8-East Room 101, New York, NY 10032. E-mail: mo2130{at}cumc.columbia.edu
The authors have disclosed no conflicts of interest.
- Copyright © 2023 by Daedalus Enterprises
REFERENCES
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.
- 26.↵
- 27.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.
- 33.↵
- 34.↵
- 35.
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- 64.↵
- 65.↵
- 66.↵
- 67.↵
- 68.↵
- 69.↵
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.↵
- 75.↵
- 76.↵
- 77.↵
- 78.
- 79.↵
- 80.
- 81.
- 82.