Abstract
BACKGROUND: Currently, no clinical or animal studies have been performed to establish the relationship between airway humidification and mechanical ventilation-induced lung inflammatory responses. Therefore, an animal model was established to better define this relationship.
METHODS: Rabbits (n = 40) were randomly divided into 6 groups: control animals, sacrificed immediately after anesthesia (n = 2); dry gas group animals, subjected to mechanical ventilation for 8 h without humidification (n = 6); and experimental animals, subjected to mechanical ventilation for 8 h under humidification at 30, 35, 40, and 45°C, respectively (n = 8). Inflammatory cytokines in the bronchi alveolar lavage fluid (BALF) were measured. The integrity of the airway cilia and the tracheal epithelium was examined by scanning and transmission electron microscopy, respectively. Peripheral blood white blood cell counts and the wet to dry ratio and lung pathology were determined.
RESULTS: Dry gas group animals showed increased tumor necrosis factor alpha levels in BALF compared with control animals (P < .05). The tumor necrosis factor alpha and interleukin-8 levels in the BALF reached baseline levels when the humidification temperature was increased to 40°C. Scanning and transmission electron microscopy analysis revealed that cilia integrity was maintained in the 40°C groups. Peripheral white blood cell counts were not different among those groups. Compared with control animals, the wet to dry ratio was significantly elevated in the dry gas group (P < .05). Moreover, humidification at 40°C resulted in reduced pathologic injury compared with the other groups based on the histologic score.
CONCLUSIONS: Pathology and reduced inflammation observed in animals treated at 40°C was similar to that observed in the control animals, suggesting that appropriate humidification reduced inflammatory responses elicited as a consequence of mechanical ventilation, in addition to reducing damage to the cilia and reducing water loss in the airway.
Introduction
In healthy individuals, the upper respiratory tract provides a warm, humid environment that plays a nonspecific defense function and facilitates the mucociliary movement of the normal airway epithelium. However, when an artificial airway is established, such as in the case of critically ill patients, the upper respiratory tract loses this critically important humid environment. Furthermore, the delivery of dry gas through an artificial airway into the lower respiratory tract can directly lead to adverse complications, including ciliary dyskinesia, respiratory tract epithelial cell damage, and increased incidence of ventilator-associated pneumonia.1,2
Although mechanical ventilation can provide life support to critically ill patients, it may also cause or aggravate lung injury. The lung tissue is susceptible to inflammation-mediated damage that can cause organ dysfunction and even multiple organ dysfunction syndrome.3–5 Infants are particularly susceptible to mechanical ventilation-mediated tissue damage, especially in the context of systemic inflammatory responses.6 Others have demonstrated that mechanical ventilation can lead to changes in lung pressure and tension, resulting in inflammatory cytokine release, alveolar edema, physiologic alveolar damage, increased alveolar permeability, and alveolar collapse.5 However, it has been demonstrated that sufficient airway humidification can reduce the incidence of ventilator-associated pneumonia, the inflammation of the nasal mucous membranes, and the incidence of postoperative pulmonary complications.7–10 Todd and John11 confirmed that airway humidification during mechanical ventilation prevented or reduced acute or chronic lung damage. To date, however, research related to ventilator-associated lung injury in animals has not incorporated the effect of humidification in the disease process,12,13 particularly since general anesthesia is administered to humans in the absence of humidification.14,15 For this reason, no animal or clinical studies have been performed to establish the relationship between airway humidification and mechanical ventilation-induced lung inflammatory responses.
Previous studies have evaluated pulmonary airway damage during invasive mechanical ventilation using different degrees of humidification in normal lungs. However, little data exist regarding the impact of humidification and the inflammatory response elicited in lungs following mechanical ventilation. Therefore, the aim of this study was to determine the relationship between airway humidification and mechanical ventilation-induced inflammatory responses. Using a rabbit model, we determined the effects of mechanical ventilation under conditions of varying temperature and humidity on the inflammatory cytokines present in the bronchoalveolar lavage fluid (BALF) and other parameters.
QUICK LOOK
Current knowledge
In healthy individuals, the upper respiratory tract warms and humidifies gas and facilitates the mucociliary movement of the normal airway epithelium. However, when an artificial airway is established, the upper respiratory tract is bypassed. The delivery of dry gas through an artificial airway into the lower respiratory tract can directly lead to adverse complications, including ciliary dyskinesia, respiratory tract epithelial cell damage, and increased incidence of ventilator-associated pneumonia.
What this paper contributes to our knowledge
Airway humidification in rabbits reduced mechanical ventilation-associated inflammatory responses, epithelial cell cilia damage, and airway water loss. The maintenance of optimal humidification levels may decrease the incidence of ventilator-induced lung injury. Adequate humidification based on duration of exposure and patient disease remains to be determined.
Methods
Care, use, and treatment of all animals in the present study were in strict agreement with the institutionally approved protocol according to the United States Public Health Service Guide for the Care and Use of Laboratory Animals. This study was approved by the Harbin Medical University Animal Care and Use Committee. Male Japanese white rabbits (n = 40) were purchased from the Animal Experiment Center of Harbin Medical University and allowed to acclimate for 7 days before the experiments were carried out. Rabbits had free access to food and water.
Rabbits were anesthetized with pentobarbital sodium (3%, 30 mg/kg), which was delivered via the auricular vein. Control group (n = 2) animals were sacrificed after anesthesia, and their tissues were used as a reference for comparison with tissues harvested from treated animals. Anesthetized rabbits were maintained in the supine position and intubated via tracheotomy using a 4.5# endotracheal tube. The intubation depth was 2 cm above the tracheal carina. The right femoral artery was cannulated to monitor the hemodynamics (BeneView T8, Mindray, Shenzhen, China) and to draw the blood specimen for the complete blood count and blood gas analyses.
All 38 rabbits (excluding those in the control group) were mechanically ventilated (Evita 4, Drager, Lübeck, Germany) for 8 h with a tidal volume of 10 mL/kg, breathing frequency of 35–50 breaths/min, PEEP of 0 cm H2O, inspiratory to expiratory ratio of 1:1.5, and FIO2 of 30% with a standard pediatric breathing tube (306/6948, Tyco, China). Normal saline was then transfused (5 mL/kg/h), and anesthesia was maintained with pentobarbital (3%, 20 mg/kg intravenously every 2 h) via the auricular vein. Muscle relaxation was maintained with pipecuronium bromide (0.04 mg/kg intravenously every 30 min). The rectal temperature was maintained at 37.0–39.0°C with a heating pad. The room temperature was maintained at 22–30°C. During the experimental procedure, the temperature and humidity in the breathing tube were monitored using a temperature and humidity probe (diameter: 5 mm) at test points 1–5 (T1–T5), and tracheal carina (using a temperature probe, diameter: 3 mm) at T6 was monitored every 30 min (JCJ508, JUCSAN, China). The probe was inserted into the tracheal carina by the tracheostomy tube. The equipment setup and test points (T1–T6) are described in Figure 1.
Equipment setup. The temperature and humidity of inspired and expired air were measured by fast-response temperature and humidity probes, which were directly attached to the breathing tube. Temperature and humidity recordings along the breathing tube were made at positions T1–T6. T1, humidifier entrance; T2, humidifier export; T3, expiratory port; T4, inspiratory port; T5, inspiration and expiration mixing section; T6, tracheal carina.
The 38 rabbits were randomly divided into 5 groups. The dry gas group (n = 6) received mechanical ventilation without humidification. Four experimental groups (32 rabbits total, n = 8/temperature) received mechanical ventilation under humidification at 30, 35, 40, and 45°C (30, 35, 40, and 45°C groups, respectively). The different temperatures correspond to the temperature at the Y-piece of the inspiratory port (T4). The temperature of the heated humidifier (MR410, Fisher & Paykel, Auckland, New Zealand) was adjusted to ensure constant temperature and humidity at T4. Except for the 30°C group, the length of the breathing tube from T2 to T4 was 56 cm. Because the temperature at T4 in the 30°C group was close to room temperature (with the temperature of the heated humidifier adjusted to the lowest setting), the temperature at T4 was higher than the predicted value of 30°C. Therefore, the length of breathing tube from T2 to T4 in the 30°C group was increased by 14 cm.
Specimen Collection and BALF Cytokine Measurements
At the end of the experiment, animals were sacrificed by exsanguination under anesthesia. The lungs were removed, the right main bronchus was tied, and the left lobe was lavaged 3 times with 10 mL/kg normal saline at 4°C to collect the BALF, which was centrifuged immediately at 3,000 rpm for 15 min at 4°C. Supernatants were collected and stored at −20°C for < 1 month before analysis. The concentrations of tumor necrosis factor alpha (TNF-α) and interleukin-8 in BALF samples were measured with an enzyme-linked immunosorbent assay kit, as described by the manufacturer (Bio-Swamp Life Science, Wuhan, China).
Scanning and Transmission Electron Microscopy Analyses of the Tracheal Epithelium
Three specimens from animals in each experimental group and 2 specimens from the control group were randomly selected for microscopic analyses. All samples were selected from the right main bronchus at 1 cm under the tracheal carina. The samples were flushed with normal saline, placed in 2.5% glutaraldehyde for 2–4 h, and examined by both a scanning electron microscope (S-3400N, Hitachi, Tokyo, Japan) and a transmission electron microscope (H7650, Hitachi).
White Blood Cell Counts
Arterial blood (2 mL) was drawn after anesthesia and before exsanguination. White blood cell (WBC) counts were determined by a clinical laboratory.
Wet to Dry Ratio Measurements
After the animals were sacrificed, the right upper lobe of the lung was harvested, weighed, placed in an 80°C drying box (101A-1, NanTong SanSi Electromechanical Science and Technology, Shanghai, China) for 48 h, and then weighed again. The wet to dry (W/D) ratio was determined from these measurements.
Histologic Examination and Grading
The right lower lobes of the lungs were fixed in formalin, embedded in paraffin, and stained with hematoxylin and eosin. Sections were examined microscopically in a blinded fashion by a pathologist. Each of the following criteria was graded on a scale from 0 to 4, where 0 = normal, 1 = minimal change, 2 = mild change, 3 = moderate change, and 4 = severe change: (1) neutrophil infiltration, (2) airway epithelial cell damage, (3) interstitial edema, (4) hyaline membrane formation, and (5) hemorrhage. Histologic scores were added for each criterion analyzed as described previously.16
Data Analysis
Continuous data were expressed as the mean ± SD. Comparisons of results before and after the experiment were made by using the paired t test for available paired design data. Comparisons of results between 2 groups were made with a 2-sample independent t test. Comparisons between multiple groups were made with a one-way analysis of variance, and the Student-Neuman-Keuls method was used for post hoc multiple comparisons. Histologic score analysis was conducted using the Kruskal-Wallis test. Test results with P < .05 were considered statistically significant. All calculations were carried out with the SAS 9.1 (SAS Institute, Cary, North Carolina).
Results
Temperature and Humidity Values at Each Test Point
Temperature, absolute humidity, and relative humidity were recorded at 6 different positions (T1 for humidifier entrance, T2 for humidifier export, T3 for expiratory port, T4 for inspiratory port, T5 for inspiration and expiration mixing section, and T6 for tracheal carina) (Table 1). With increasing humidification temperature, the temperature, absolute humidity, and relative humidity values gradually increased compared with values in the dry gas group. In the 40°C group, the actual temperature in the Y-piece reached 39.0 ± 1.90°C, the absolute humidity was 47.3 ± 3.95 mg/L, and the relative humidity was 95.6 ± 0.48%. The corresponding values in the tracheal carina were 38.2 ± 0.71°C, 39.9 ± 2.72 mg/L, and 87.2 ± 6.43%, respectively (Table 1).
Mean Values of Temperature, Absolute Humidity, and Relative Humidity Measured at Each Hour for 8 h at Test Points 1–6 (T1–T6)
Inflammatory Cytokine Concentrations in BALF
The TNF-α levels in the dry gas group (71.1 ± 6.92 pg/mL) were increased compared with levels in the control group (52.5 ± 6.74 pg/mL, P < .05), whereas the TNF-α and interleukin-8 concentrations decreased with increasing temperature, especially in the 40 and 45°C groups. The inflammatory cytokine concentrations in the 40 and 45°C groups were similar to levels observed in the control group (Table 2).
Interleukin-8 and TNF-α Concentrations (pg/mL)
Electron Microscopy Analyses
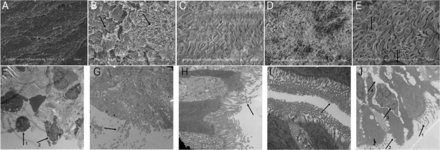
According to the scanning electron microscope analysis of the tracheal cilia integrity, the dry gas group animals demonstrated almost no cilia compared with the 30°C group, which showed few cilia, lodging, and adhesion. Animals in the 35°C group had no cilia defects but displayed lodging and adhesion. Animals in the 40°C group presented with nearly normal tracheal cilia and no additional defects. Animals in the 45°C group showed some damage to the cilia integrity in addition to lodging and adhesion (Fig. 2).
Scanning electron micrographs (A–E) and transmission electron micrographs (F–J) of tracheal epithelial structures. A: dry gas group devoid of cilia. Magnification ×1,000. B: 30°C group. Arrow 1 indicates loss of cilia integrity, adhesions, and lodging. Arrow 2 indicates part of a detached cilium. Magnification ×1,000. C: animal in the 35°C group displaying loss of cilia integrity, adhesions, and lodging, with cilia remaining attached. Magnification ×2,000. D: animal in the 40°C group showing nearly normal tracheal cilia. Magnification ×1,000. E: 45°C group. Arrow 1 indicates part of an unattached cilia. Arrow 2 indicates an irregularly arranged cilia with adhesions and lodging. Magnification ×2,000. F: dry gas group. Arrow 1 indicates inflammatory infiltrates. Arrow 2 indicates unattached tracheal epithelial cell cilium and regions devoid of cilia. Magnification ×8,000. G: 30°C group. The arrow indicates decreased cilia density in the trachea. Magnification ×7,000. H: 35°C group. The arrow indicates decreased cilia density in the trachea. Magnification ×8,000. I: 40°C group. Shown are nearly normally arranged cilia in the tracheal epithelium. Magnification ×10,000. J: 45°C group. Arrow 1 indicates decreased cilia density. Arrow 2 indicates cytoplasmic overflow. Arrow 3 indicates inflammatory infiltrates. Arrow 4 indicates water in the interstitial space. Magnification ×6,000.
Transmission electron microscope analysis of the tracheal epithelium demonstrated that most of the epithelium in the dry gas group was missing. The dry gas group tracheal epithelium also showed inflammatory cell infiltrates and subepithelial structural damage. Animals in the 30°C group displayed some epithelial cell loss but no organelle damage. Animals in the 35°C group showed a regular array of tracheal cilia, preserved tight junctions, and intact organelles, with only a few cells with minor cilia damage. Animals in the 40°C group showed a regular array of abundant tracheal cilia and intact organelles. Animals in the 45°C group demonstrated some cilia damage, cell degeneration, edema, damaged organelles, loss of tight junction integrity, and inflammatory cell infiltration (Fig. 2).
WBC Counts
No significant differences in the WBC counts were observed between the dry gas group and the other experimental groups (P > .05); the average peripheral WBC count in the dry gas group was 1.28 ± 5.75 × 1010, and in the 45°C group, it was 1.3 ± 6.66 × 1010 (Table 3).
White Blood Cell Changes Before and After Mechanically Administered Ventilation
W/D Ratio
The W/D ratio of the dry gas group (4.96 ± 0.1) was lower than that of the control group (5.24 ± 0.03, P < .05). No significant differences in the W/D ratio were observed between the dry gas group and animals in the other groups (Table 4).
Wet to Dry Ratio in All Groups
Histologic Analyses
To compare pathologic differences in the context of different humidification conditions, we monitored histologic changes microscopically (Fig. 3) and compared histologic scores. As shown on Table 5, the histologic scores for animals in the 40°C group were significantly lower than values observed for the dry gas group and the 45°C group (P < .05).
Hematoxylin and eosin staining of lung tissues. A: dry gas group. Arrow 1 indicates inflammatory infiltrates, and arrow 2 indicates alveolar collapse. Magnification ×100. B: 30°C group. Arrow 1 indicates inflammatory infiltration, arrow 2 indicates alveolar hemorrhage, and arrow 3 indicates alveolar collapse. Magnification ×100. C: 35°C group. Arrow 1 indicates interstitial edema, and arrow 2 indicates inflammatory infiltration. Magnification ×400. D: 40°C group. Inflammatory cell infiltration was absent. Magnification ×200. E: 45°C group. Arrow 1 indicates inflammatory infiltrates, arrow 2 indicates alveolar collapse, and arrow 3 indicates alveolar hemorrhage. Magnification ×200. F: control group. Arrow 1 indicates alveolar hemorrhage, and arrow 2 indicates alveolar collapse. Magnification ×200.
Lung Histology Scores
Discussion
Clinical guidelines suggest that mechanical ventilation devices provide a humidity level between 33 and 44 mg/L H2O and gas temperatures between 34 and 41°C at the circuit Y-piece with a relative humidity of 100%. Our results using a rabbit model supported these recommendations, demonstrating that a temperature of 40°C at the circuit Y-piece was most effective.17 In the 40°C group, we observed decreased lung inflammation and intact tracheal cilia compared with the other conditions tested, which confirmed clinical findings and provided the supporting evidence for clinical guidelines.
The TNF-α concentrations in the BALF collected from animals in the dry gas group were significantly elevated compared with the levels observed in the control group BALF. As the temperature increased, the TNF-α and interleukin-8 concentrations in the corresponding experimental groups decreased, especially in the 40 and 45°C groups, which had values significantly lower than those in the dry gas group. Therefore, we conclude that mechanical ventilation elicited inflammatory responses, but humidification at 40 and 45°C significantly reduced the inflammatory cytokine levels.
Imai et al18 demonstrated that mechanical ventilation caused ventilator-induced lung injury, due mainly to the release of inflammatory cytokines in the alveoli. Tremblay et al19 ventilated rat lungs for 2 h without humidification and found that the concentrations of TNF-α and interleukin-6 were significantly increased. Others have demonstrated that low tidal volumes during mechanical ventilation result in reduced lung capacity and pulmonary inflammation.20–23 However, Koutsourelakis et al8 found that the concentrations of inflammatory cytokines in nasal lavage fluids were reduced when noninvasive ventilation with humidification was used.
Scanning electron microscopy analyses of the trachea demonstrated that almost all of the cilia were absent under non-humidified conditions. In the 40°C group, the tracheal cilia levels were similar to physiologic levels. However, under- or overhumidification resulted in reduced cilia levels, lodging, and adhesion. Studies have shown that the delivery of gas into the lower respiratory tract without humidification not only caused loss of cilia but changes to cilia morphology and movement.24 Chidekel et al25 demonstrated that exposure to reduced humidity levels reduced epithelial cell function and increased inflammation in the environmental chambers of airway epithelial monolayers.
Transmission electron microscopy analysis of the tracheal epithelium revealed well-defined infiltration of inflammatory cells in animals from the dry gas and 45°C groups. The degree of inflammation was accompanied by increased inflammatory cytokines and WBC counts in the dry gas group BALF. In contrast, inflammatory BALF cytokine levels were not associated with the level of inflammatory cell infiltrates or increased WBC counts observed in the 45°C group. This lack of association was probably due to overhumidification or burn damage to the respiratory tract associated with the 45°C group animals. These animals probably experienced overhumidification so that increased water levels in the alveoli facilitated cytokine diffusion into the interstitial fluid. Another reason may be differences in the cell degeneration thresholds between the 40 and 45°C groups. There were more cellular changes observed in the 45°C group in addition to a coiling effect caused by the inhaled gas. Therefore, although there was only a 0.6°C change at the tracheal carina between these 2 groups, this temperature difference may have been sufficient to cause changes to the respiratory tract epithelium. Modell et al14 found that overhumidification not only caused pulmonary edema but also elicited inflammatory responses. Relatively minor temperature and humidity changes also affected the outcome during mechanical ventilation; this response may be due to the highly sensitive nature of the tracheal epithelium. Both under- and overhumidification caused harm to the tracheal epithelium, especially to the cilia. Therefore, the correct degree of humidification must be utilized to prevent inflammatory responses from being elicited.
Although the WBC counts did not exceed normal physiologic values, rabbits in the dry gas groups and 45°C groups presented with WBC counts that exceeded 1 × 1010. Villar and Blanco15 found that mechanical ventilation-induced lung injury stimulated the production of inflammatory mediators that led to local or systemic inflammatory reactions. Lung inflammation due to mechanical ventilation-induced injury could easily lead to increased levels of inflammatory cytokines, damage to the alveolar and capillary epithelial cells, and, eventually, ARDS. The use of appropriate humidification treatment can reduce the severity of the inflammatory response. The data presented in this study further suggest that optimal humidification may decrease the incidence of ventilator-induced lung injury during mechanical ventilation.
Compared with animals in the control group, the dry gas group animals presented with significantly reduced W/D ratios. This finding suggested that the animals experienced significant loss of moisture and heat as a result of mechanical ventilation in the absence of humidification. Gross and Park26 found that gas administration into the lower respiratory tract without proper humidification was associated with loss of moisture and heat, cilia damage, sputum retention, pulmonary infection, and atelectasis, especially in newborns. The histologic scores for animals in the 40°C group were significantly lower than scores observed for animals in the dry gas groups and 45°C groups, suggesting that either reduced or elevated humidification levels could cause airway injury,14,24,25 which was consistent with the observed changes in inflammatory cytokines and tracheal cilia integrity.
We used 10 mL/kg as a normal tidal volume,27 but previous work demonstrated that low mechanical ventilation tidal volumes also could result in reduced lung capacity and pulmonary inflammation.20–22 Koutsourelakis et al8 found a reduction in the levels of inflammatory cytokines in nasal lavage fluids following the administration of noninvasive mechanical ventilation with humidification. This suggested that the administration of mechanical ventilation without humidification represents one of the major factors affecting ventilator-associated lung injury that is not only caused by mechanical injury but also caused by insufficient humidification, resulting in inflammation. It is therefore important to determine whether mechanical ventilation with normal tidal volumes and optimal humidification can be used to prevent or reduce ventilator-associated lung injury.
Chalon et al9,28 found that subjects ventilated without humidification for > 1 h during the course of general anesthesia presented slight damage to the respiratory tract epithelium. Ventilation for > 3 h resulted in changes to the cytoplasm of the tracheal epithelial cells in about 30% of subjects, which were accompanied by changes to the nuclei in about 48% of subjects. However, subjects who received humidification therapy during mechanical ventilation did not present with any significant changes to their epithelial cell structure. Previous studies29,30 showed that, during general anesthesia, the delivery of cold, dry gas into the lower respiratory tract resulted in pulmonary surfactant changes, increased pulmonary surface tension, and alveolar collapse. Based on the data presented in this report, we recommend that patients receiving general anesthesia also receive humidification therapy.
The beneficial effects associated with airway humidification depend on various factors, including the temperature of the inhaled air, room temperature, ventilation, type of humidification device used, and dimensions (length and diameter) of the breathing tube. In our study, except for minor fluctuations in the room temperature and the length of the breathing tube in animals in the 30°C group, all other conditions remained constant. When the breathing tube length was increased by 10 cm, the temperature decreased by 1.5–2.0°C, the absolute humidity decreased by about 2 mg/L, and the relative humidity increased. Inui et al31 found that when the length of the breathing tube was increased by 10 cm, the temperature was decreased by 1.0–2.0°C, and absolute humidity was decreased by about 1–2 mg/L, with a corresponding increase in relative humidity. Their findings are consistent with the work presented in this report.
The data presented by us and others suggest that optimal humidification levels need to be maintained during mechanical ventilation as a means of preventing damage to the airway epithelium. Furthermore, factors that can affect humidification efficacy, such as the tidal volume, humidification device, room temperature, and tubing length, should be considered. In addition, prewarming the air before it enters the breathing tube,32 insulating the breathing tube, and monitoring the humidity levels should be included as part of the treatment process. Moreover, the temperature and humidity sensor was too big to use in small animals like mice; therefore, additional studies should be conducted in larger animals to further expand on the present findings.
Conclusions
Airway humidification in rabbits reduced mechanical ventilation-associated inflammatory responses, epithelial cell cilia damage, and airway water loss. The maintenance of optimal humidification levels may decrease the incidence of mechanical ventilation-associated ventilator- induced lung injury. Therefore, it is recommended that patients receiving general anesthesia also receive humidification therapy.
Acknowledgments
We thank Medjaden Bioscience Limited for assisting in the preparation of the manuscript.
Footnotes
- Correspondence: Hai-Bo Li MD, Department of ICU-D, Second Affiliated Hospital of Harbin Medical University, Anesthesiology and Critical Medicine Major Laboratory of Heilongjiang Province, Harbin, Heilongjiang 150081, China. E-mail: mzkicu{at}126.com.
The authors have disclosed no conflicts of interest.
See the Related Editorial on Page 1854
- Copyright © 2015 by Daedalus Enterprises