Abstract
BACKGROUND: Bench and clinical data indicate that techniques for applying noninvasive respiratory support may vary in terms of effectiveness, application, and tolerability. We implemented a new nasal interface and flow-generation system for the delivery of noninvasive respiratory support (NRS) to replace previously used systems. Our goal was to determine whether there were significant differences in clinically relevant outcomes between our new method and conventional systems.
METHODS: We conducted a prospective observational study of preterm infants requiring noninvasive respiratory support during our initial implementation of a new nasal interface (RAM), and compared these data with a historic control group. Demographic, baseline, and clinical outcome data were collected. Clinical outcomes and comorbid conditions were compared by using the chi-square test for categorical information and the Student t test or Wilcoxon rank-sum test for quantitative data, depending on normality testing when using the Shapiro-Wilk test. Uni- and multivariate logistic regression were conducted to determine predictive factors for the development of bronchopulmonary dysplasia.
RESULTS: There were no significant group differences in important comorbid conditions, invasive mechanical ventilation days (P = .16), or NRS failure within the first 7 d after birth (P = .10). Although there were no significant differences in the use of CPAP or noninvasive ventilation, settings with were significantly higher (P < .001) in the RAM group. There were more incidences of retinopathy of prematurity (P = .02) post RAM implementation, and the time to first reintubation was significantly shorter in the RAM group (P = .044). However, there were significant reductions post RAM in total days on any respiratory support (P = .009), total NRS days (P = .02), and supplemental O2 duration (P = .02). There was a trend toward reductions in bronchopulmonary dysplasia rates (P = .053), and the incidence of device-related tissue breakdown was significantly reduced (P < .001) post RAM. Multivariate logistic regression results showed the type of system (odds ratio [OR] 0.19, 95% CI 0.04–0.87; P = .032) and total invasive ventilation time (OR 0.94, 95% CI 0.89–0.99; P = .02) were predictors for the development of bronchopulmonary dysplasia.
CONCLUSIONS: The ability to apply continuous distending pressure through consistent application of NRS with the RAM cannula attached to a ventilator may improve clinical outcomes, including the duration of respiratory support and pressure-ulcer rates. The influence of this system on the development of bronchopulmonary dysplasia and the significantly increased retinopathy of prematurity requires further study.
- preterm infant
- extremely low birthweight (ELBW)
- very low birthweight (VLBW)
- noninvasive respiratory support
- nasal CPAP
- noninvasive ventilation
- high-flow nasal cannula
- bronchopulmonary dysplasia
- pressure ulcer
Introduction
Preterm low birthweight neonates are at high risk for pulmonary complications and need respiratory support secondary to immature lung development.1 One of the main goals in treating respiratory distress syndrome is to establish and maintain normal functional residual capacity.2 Surfactant dysfunction makes alveoli unstable and prone to collapse, and repeated de-recruitment from variable pressure applications may lead to atelectrauma and ventilator-induced lung injury.3,4 Analysis of research results indicated that providing optimal functional residual capacity can protect against lung injury and reduce required FIO2.3
Early noninvasive respiratory support is frequently used in this population, to replace invasive mechanical ventilation for alveolar recruitment and reduce the risk of lung injury and infection.1 Physiologic benefits of standard noninvasive respiratory support modes, such as, CPAP, include improved oxygenation and ventilation-perfusion matching secondary to increased functional residual capacity.5 There are studies that indicate that systems used to deliver noninvasive respiratory support can potentially influence these clinical outcomes.6 These systems vary in pressure transmission, imposed work of breathing, and the ability to recruit the lung, depending on a variety of factors, such as ventilator settings, flow characteristics, and percent leak.7 For example, the Infant Flow Driver (Infant Flow LP Nasal CPAP System, Vyaire, Cape Town, South Africa) was developed to reduce expiratory resistance and fluctuations in airway pressure, but preliminary studies were mixed regarding benefits of this device over other interfaces.8
Studies also indicated that noninvasive respiratory support interfaces, including the one used by the Infant Flow Driver system, present a risk for the development of nasal injury.9,10 These devices have a range of manufacturer-recommended attachment methods and are made from materials with diverse durometers. Configuration differences lead to variances in application time, patient tolerance, and the use of each device with various noninvasive respiratory support modes. Use of only one type of interface, mask or prongs, frequently leads to skin breakdown, therefore, the literature9,11 recommends scheduled interface rotations as this technique has been associated with reduced incidence of nasal injury.12 The need to rotate interfaces to avoid pressure ulcers necessitates breaking the circuit and, thus, losing pressure. Research has shown that sustained distending pressure is crucial to recruiting and maintaining functional residual capacity.6
The RAM cannula (Neotech, Valencia, California) received FDA clearance to deliver high- or low-flow oxygen, and, off label, can deliver nasal CPAP and noninvasive ventilation (NIV). This system may allow earlier application, along with more stable use and pressure transmission compared with conventional noninvasive respiratory support systems. There are few comparative studies of noninvasive respiratory support interfaces combined with different flow-generation systems and no published clinical outcome data to date that compared these with the RAM cannula in very low birthweight (< 1,500 g) and extremely low birthweight (< 1,000 g) infants. Analysis of bench data indicate that there are differences in pressure transmission between the RAM and other cleared interfaces, with lower delivered pressure with the RAM.13–15 Clinically, only one observational study was found that reviewed a single center's experience when substituting the RAM for a conventional noninvasive respiratory support interface using 2 ventilators with different flow-generation characteristics.16 The investigators used the RAM to reduce the impact of tissue breakdown and agitation that resulted from conventional noninvasive respiratory support interfaces and found that most infants were successfully weaned by using the RAM cannula with NIV or CPAP.16 However, this study did not report outcomes with the RAM compared with other interfaces or between ventilators.
Our goal was to determine whether there were significant differences in clinically relevant outcomes between conventionally used devices (the Infant Flow Driver system and another approved high-flow nasal cannula [HFNC]; control) and the RAM cannula (used with a pediatric ventilator to deliver CPAP and/or NIV and as an HFNC [RAM]) in this patient population. We hypothesized that the RAM could improve outcomes, including rates of bronchopulmonary dysplasia and skin breakdown compared with conventional systems for the delivery of noninvasive respiratory support.
QUICK LOOK
Current knowledge
Methods used to deliver noninvasive respiratory support vary in terms of effectiveness, application, and tolerability. The non-invasive system and interface can impact impact delivered pressure, leak compensation, and patient comfort. All of these factors influence the success and complications of these modalities.
What this paper contributes to our knowledge
Use of the RAM cannula attached to a pediatric ventilator may improve clinical outcomes, including duration of respiratory support and skin breakdown in low birthweight infants with respiratory distress syndrome. Use of the cannula appeared safe as there were no significant differences in most comorbidities. However, the rise in retinopathy of prematurity should be investigated further. The influence of a high-flow nasal cannula system on the development of bronchopulmonary dysplasia requires further study.
Methods
We conducted a prospective observational study of infants < 29 weeks' gestational age and < 1,500 g born in our level III neonatal ICU and who required noninvasive respiratory support from 2014 to 2015 during our initial implementation of the RAM cannula (n = 36) and compared these data with an historical control group placed on conventional systems (n = 36) from 2012 to 2013. Exclusion criteria included any infant who died before the initiation of noninvasive respiratory support or was transferred before 36 weeks' gestational age. The study was approved by MedStar's institutional review board (2015–010).
All mothers who deliver infants < 34 weeks' gestational age are given betamethasone per our standard antenatal protocol based on American Congress of Obstetricians and Gynecologists guidelines.17 Infants are resuscitated immediately post birth per American Academy of Pediatrics/American Heart Association's Neonatal Resuscitation Program guidelines18 by using the Neopuff (Infant T-Piece Resuscitator, Fisher & Paykel, Auckland, New Zealand) with pressures of 20–25 cm H2O and PEEP of 5 cm H2O. The FIO2 was provided based on Neonatal Resuscitation Program guidelines,18 and titrated to achieve targeted preductal saturations for time post birth. Our neonatal ICU uses a hybrid of the INtubate-SURfactant-Extubation method,19 with intubation, early surfactant delivery, and limited invasive mechanical ventilation, when possible. Preterm infants at < 29 weeks' gestation are immediately intubated at birth, given surfactant, then extubated to noninvasive respiratory support within 24 h under a standardized protocol if clinical criteria are met. This protocol and criteria for extubation (see the supplementary materials at http://www.rcjournal.com) were unchanged between the study periods. Caffeine citrate was given immediately post extubation before and after RAM implementation and then continued daily.
Infants who did not meet extubation criteria or who were re-intubated were maintained on invasive mechanical ventilation until clinically stable for noninvasive respiratory support. Pressure control was the initial and maintenance mode of invasive ventilation (Babylog 8000, Dräger, Lubeck, Germany), with high-frequency oscillatory ventilation (Vyaire) used as rescue in patients for whom this mode failed. Normocapnia or permissive hypercapnia (PCO2 45–65 mm Hg) was targeted for invasive ventilation, depending on the time frame post birth. Oxygenation goals for all respiratory support modes were guided by protocol based on the 2012 American Academy of Pediatrics' guidelines for perinatal care.20 Incubator humidity and the use of developmental positioning was unchanged between the study periods, as was the overall clinical management of our preterm infants.
Noninvasive Respiratory Support Systems and Modes
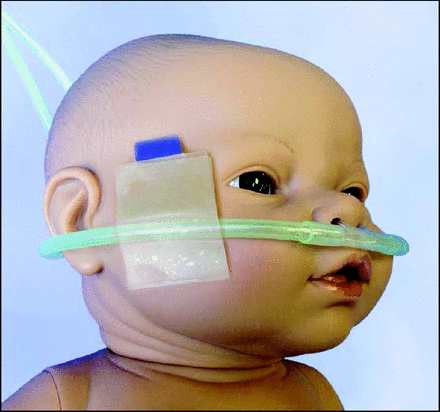
We implemented the RAM system (Fig. 1) in 2014 as a new nasal interface for the delivery of noninvasive respiratory support in our neonatal ICU, excluding low-flow supplemental O2. This device replaced previous interfaces used for the delivery of CPAP, NIV, and HFNC. The RAM cannula was sized, according to the manufacturer's recommendations, as micropreemie or preemie to approximate naris occlusion of 80%, with an outer diameter of 3 mm for both sizes (septal distance varied). We attached the RAM cannula using the EZ-hold Tubing and Cannula Holder or DuoDERM (ConvaTec, Oklahoma City, Oklahoma), and white cloth tape bilaterally to the cheek areas. The Babylog 8000, a constant-flow, time-cycled ventilator, was used as the flow-generation system for CPAP and NIV, while HFNC was provided via an external flow meter attached to 50 psi oxygen.
The RAM cannula (Neotech, Valencia, California) is FDA cleared for high- and low-flow oxygen therapy. Off label, the interface can also be used to deliver noninvasive ventilation and CPAP.
The Infant Flow LP Nasal CPAP System for CPAP and NIV (Vyaire) (Fig. 2), including short bi-nasal prongs and mask, were fitted according to the prong and mask sizing guide and attached by using equipment included in the packaging. DuoDERM nasal, upper-lip, and forehead cutouts were used as a preventive dressing for pressure-point areas to minimize soft-tissue damage. These interfaces were rotated every 4–8 h, depending on skin inspection, with support provided to the circuit to minimize weight and torque placed on vulnerable areas, such as the nose and forehead. These interfaces are proprietary to the Infant Flow SiPAP (Vyaire), a variable, fluidic-flow opposition device, which was used to deliver both CPAP and NIV in this group. The conventional interface for HFNC (Westmed Comfort Soft Plus Cannula High Flow, Tucson, Arizona) was provided in the “premature” (∼3-mm outer diameter) size because there were no sizing guidelines. This interface was attached to the bilateral cheek areas with DuoDERM and white-cloth tape. Flow was provided by using an external flow meter attached to 50 psi oxygen. A chin strap was not used to close the mouth with any interface studied.
Conventional interfaces for the delivery of noninvasive respiratory support in neonates. Nasal mask (A) and nasal prongs (B).
The primary mode of noninvasive respiratory support typically used for both groups was CPAP, starting at 5 cm H2O. Bi-level NIV, starting at an inspiratory positive airway pressure of 10 cm H2O and an expiratory positive airway pressure of 5 cm H2O, was typically instituted secondarily before re-intubation in infants for whom CPAP failed and less frequently as the initial noninvasive respiratory support mode. HFNC (Infant Respiratory Care System, Fisher & Paykel) was used as transitional therapy (see the supplementary materials at http://www.rcjournal.com) until a flow of <2 L/min was indicated, then the subjects were weaned to supplemental O2 via a standard nasal cannula (AirLife Instant Cushion Nasal Cannula, CareFusion, Yorba Linda, California).
Data Collection and Analysis
Demographic, baseline, and clinical outcome data were obtained using standardized collection formats and were entered into a secure database. Data for the historical control were extracted from our electronic medical record system into Excel (Microsoft, Redmond, Washington); prospectively collected information for the RAM group was observed and recorded on neonatal ICU flow sheets, with additional data obtained when necessary from medical records during the study period. Demographic and baseline data included gestational age, weight, sex, race, CRIB II (Clinical Risk Index for Babies) scores, 5-min Apgar score, the presence of chorioamnionitis or premature rupture of membranes, the number of caesarean sections, and use of postnatal steroids. Clinical outcomes during hospitalization, such as invasive mechanical ventilation, noninvasive respiratory support settings and use, re-intubations (see the supplementary materials at http://www.rcjournal.com), supplemental O2 use, comorbid conditions (pneumothorax, necrotizing enterocolitis, patent ductus arteriosus, feeding difficulties, pulmonary hemorrhage, intraventricular hemorrhage, retinopathy of prematurity [ROP]), bronchopulmonary dysplasia (defined as the need for supplemental O2 at 36 weeks' gestation), and skin integrity (erythema, indentations, breaks to the skin) were also collected.
All continuous data are presented as either mean ± SD or median (range), depending on skew, and categorical values are presented as number (%). Comparisons between the RAM and control groups were made by using the chi-square test for categorical information and the Student t test or Wilcoxon rank-sum test for quantitative data, depending on normality testing using the Shapiro-Wilk test. Univariate logistic regression was also performed, and all variables with a P ≤ .10 were examined in a stepwise multivariate logistic regression model. Data were imported into SAS 9.4 (SAS Institute, Cary, North Carolina) and analyzed with a P < .05 delineated as statistically significant.
Results
A total of 105 patients were eligible for study inclusion (n = 60 [control group], n = 45 [RAM group]), with 33 patients excluded (Fig. 3). Twenty-four patients from the control group were excluded, including 9 infants who died before noninvasive respiratory support and 15 who were transferred. Nine infants were excluded from the RAM group, including one infant who died before noninvasive respiratory support and 8 who were transferred. Excluded patients were sicker (median [range]) (CRIB II score: control group, 14 [8–19]; RAM group, 12 [9–17]; P < .001), younger (gestational age: control group, 24+5 weeks [22+0–28+0 weeks]; RAM group, 25+2 weeks [23+0–28+5 weeks]; P < .001); and smaller (weight: control group, 670 g [470–1023 g]; RAM group, 698 g [475–1380 g]; P < .001) than the subjects included in the study. Only one infant received noninvasive respiratory support at any time before transfer in the treatment (RAM) group, whereas, in the control group, 6 infants received noninvasive respiratory support before transfer. There were more patients excluded in the control group versus the treatment group and more infants who received noninvasive respiratory support before transfer in the control group. Statistical analysis showed that these infants also had higher CRIB II scores, lower gestational age, and weighed less than infants excluded in the RAM group (all P < .001). There also were variations in our admission rates between study periods, when a District of Columbia Medicaid plan was no longer able to pay claims, starting in 2014.
Flow chart.
Baseline Status and Comorbid Conditions of Included Subjects
Thus, there were 36 subjects included in the control group and 36 in the treatment group (HFNC RAM or NIV/RAM group). There were no significant between-group differences in the number of extremely low birthweight (<1,000 g) infants, or those < 750 g. There also were no significant differences between the control and RAM groups for gestational age, weight, sex, race, CRIB II scores, 5-min Apgar, chorioamnionitis, premature rupture of membranes, or birth by caesarean section. However, there were significantly fewer steroids used post birth in the RAM group. Details of the demographic and baseline clinical information are presented in Table 1.
Demographic and Baseline Clinical Data of the Study Subjects
In addition, there were no significant between-group differences in comorbidities, such as necrotizing enterocolitis, patent ductus arteriosus, pulmonary hemorrhage, feeding difficulties, or the occurrence or grade of intraventricular hemorrhage. There were no cases of pneumothorax development in either group. However, there were more incidences of any grade ROP after RAM Cannula implementation. Although there were no significant differences in ROP severity (P = .059), with the majority stage 1 (control group, 7/12 [58%]; RAM group, 18/22 [82%]), the grade trended higher in the control group. Information on comorbidities is provided in Table 2. No infants in either study group died before discharge.
Comorbid Conditions During Hospitalization in the Control and RAM Groups
Invasive Ventilation, Extubation, and Re-intubations
There were no statistical differences in the total number of invasive ventilation courses or total duration of invasive mechanical ventilation between the groups. However, the initial duration of invasive mechanical ventilation was significantly shorter after RAM implementation (Table 3). Consistent with the latter result, 14 subjects (39%) in the control group were initially extubated within 24 h post birth to noninvasive respiratory support, whereas 23 (64%) were extubated within this time frame after RAM implementation. Although there were no significant differences in the total number of infants who required at least one re-intubation or the total number of re-intubations, the time to re-intubation after initial extubation was significantly shorter in the RAM group compared with the control group.
Clinical Outcomes in the Subjects Treated With Conventional Interfaces or RAM Cannula
All 9 infants in whom the RAM system was used were re-intubated for worsening respiratory distress (apnea, bradycardia, desaturations, and/or retractions), whereas those in the control group were re-intubated for worsening respiratory distress (2/3) and pulmonary hemorrhage (1/3). The subjects in the control group were all ≥27 weeks' gestation, with a weight of ≥950 g. However, more than half of the infants (5/9) initially re-intubated with the RAM were at ∼25 weeks' gestation, and the majority (8/9) were <750 g at birth. The number of infants re-intubated within 72 h of initial extubation with the RAM trended higher but was not statistically significantly, nor was the number of infants for whom noninvasive respiratory support failed within the first 7 d post birth. There were no between-group differences in the use of high-frequency oscillatory ventilation as rescue (P = .16).
Noninvasive Respiratory Support Modes, Settings, and Use
Noninvasive respiratory support was broken down into CPAP, NIV, and HFNC settings and use. There were no significant between-group differences in CPAP as the initial mode (P = .84), number of infants placed on CPAP (P = .15), frequency of use (P = .08), or total days on CPAP (P = .08). However, there was a significant difference (P < .001) in CPAP settings, with higher settings used after RAM implementation (before and after RAM: 5 cm H2O; control 4–7 cm H2O, RAM 5–6 cm H2O). For NIV, there were no significant between-group differences in the number of infants placed on this mode (P = .33), frequency of use (P = .15), or total days on NIV (P = .82). But, again, there were significantly higher set NIV pressures after RAM implementation for both inspiratory positive airway pressure (P < .001) (control group, 10 [10-10] cm H2O; RAM group, 10 [10-20] cm H2O), and expiratory positive airway pressure (P = .001) (control group, 5 [5-6] cm H2O]; RAM group, 5 [5-10] cm H2O). For HFNC, there were no significant between-group differences in settings (2–4 L/min, P = .20), the number of infants placed on HFNC (P = .64), frequency of use (P = .82), or total days of use (P = .09).
Clinical Outcomes
There were no significant between-group differences in the hospital length of stay (P = .67). However, there were statistically significant reductions in total days on any respiratory support, total noninvasive respiratory support days, and supplemental O2 duration after RAM implementation (Table 3). Bronchopulmonary dysplasia rates trended lower, whereas the incidence of device-related skin or mucosal breakdown was significantly reduced in subjects placed on the RAM system. Before RAM implementation, we recorded 19 subjects (42%) with skin or mucosal injuries to the nasal septum (n = 12), cheeks (n = 3), bridge of the nose (n = 5), lips (n = 1), and forehead (n = 3). After RAM implementation, we found 3 subjects (8%) with lesions to the upper lip (n = 1) and nasal septum (n = 3).
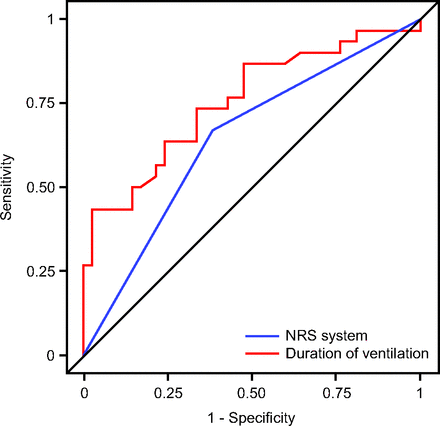
Multivariate logistic regression to predict the outcome of bronchopulmonary dysplasia included all variables with P ≤ .10 in the univariate analysis. The type of noninvasive respiratory support system used (Infant Flow Driver or RAM system), gestational age, total NIV time, total invasive ventilation time, CRIB II score, patent ductus arteriosus, postnatal steroid use, weight, and weight of <1,000 or <750 g were all entered into the model. Our analysis found that the type of system (odds ratio [OR] 0.19, 95% CI 0.04–0.87; P = .032) and total invasive ventilation time (OR 0.94, 95% CI 0.89–0.99; P = .02) were predictors for the development of bronchopulmonary dysplasia. The receiver operating characteristic analysis showed an area under the curve of 0.64 (OR 0.30, 95% CI 0.11–0.82; P = .01) for type of noninvasive respiratory support system, and area under the curve of 0.75 (OR 1.08, 95% CI 1.03–1.13; P = .001) for total invasive ventilation time (Fig. 4).
Receiver operating characteristic analysis for type of noninvasive respiratory support (NRS) system used (RAM vs Infant Flow Driver) and total time spent on invasive mechanical ventilation. Area under the curve of 0.64 (odds ratio [OR] 0.30, 95% CI 0.11–0.82; P = .01) for type of NRS system and area under the curve of 0.75 (OR 1.08, 95% CI 1.03–1.13; P = .001) for total invasive ventilation duration.
Discussion
To our knowledge, this was the first study to compare clinical outcomes for noninvasive respiratory support between conventional systems and the RAM system used with 2 noninvasive respiratory support delivery methods. We found reduced days of noninvasive respiratory support and pressure-ulcer rates when using the RAM system and demonstrated these improvements among infants with similar acuity, comorbidities, and clinical management. Our regression model showed that the RAM system corresponded to lower odds of developing bronchopulmonary dysplasia, although receiver operating characteristic analysis found that this model had relatively weak predictive ability. Not surprisingly, the total time on invasive ventilation was a better, albeit fair, predictive model. The literature indicates that reducing this variable can improve rates of chronic lung disease, however, there were no differences in invasive ventilation time between the study groups.
Nevertheless, these results are encouraging because our rates of chronic lung disease in infants at 22–29 weeks' gestation were historically higher than those reported in benchmark data.22 Before the use of the RAM Cannula, our neonatal ICU used alternating proprietary interfaces attached to a variable-flow system for the delivery of NIV and/or CPAP to avoid the development of serious tissue injury. Neither the Infant Flow Driver prongs nor mask could be safely continued for long periods in the extremely premature and extremely low birthweight population that we primarily treated. These frequent breaks to the circuit to change interfaces caused loss of pressure. The published research concerning de-recruitment centers on circuit breaks in subjects on mechanical ventilation.23–26 Most of this literature reviews the loss of PEEP after ventilator disconnection with evidence that recruited lung volume is lost and collapse occurs rapidly.27 The repetitive opening and closing of alveoli and terminal airways has been implicated in the development of significant lung injury and bronchopulmonary dysplasia.28–30 Noninvasive ventilation does not provide a complete seal within the circuit secondary to interface or mouth leaks, but, ideally, it should provide consistent pressure to maintain functional residual capacity. We speculated that maintaining distending pressure by using the RAM attached to a ventilator may have been the main factor that led to the improvements we found.
Next, although noninvasive respiratory support failure rates within the first week post birth were not significantly different between groups, the time to first re-intubation was significantly shorter in the RAM group. There were several possible explanations for this result. First, fewer infants in the control group were extubated within 24 h post birth compared with the RAM group. Although there was no change in extubation criteria between the study periods (see the supplementary materials at http://www.rcjournal.com, Table 1), this may have represented a practice change secondary to easier RAM attachment methods, with a more aggressive weaning approach and recommendations by respiratory therapists. The Infant Flow Driver system required significant time to apply, used a separate flow-generation system, and could not be used with the manual resuscitator. There also were differences in the characteristics of infants who required initial re-intubation (for re-intubation criteria, see Table 2 in the supplementary materials at http://www.rcjournal.com), with younger and smaller infants in the RAM group compared with the control group. Our initial CPAP failure rates in infants ∼25 weeks' gestation were not unexpected, although, research has shown that CPAP delivery is “progressively less successful” in smaller, more preterm infants.31 Several studies indicate that NIV improves extubation and reduces apnea of prematurity and the need for invasive mechanical ventilation in this patient population.32–35
It is also possible that set and transmitted pressures with the RAM system and constant-flow ventilator were insufficient to maintain adequate functional residual capacity. Studies are mixed regarding the Infant Flow Driver system, with several indicating reduced work of breathing and pressure fluctuations with diversion of expiratory flow at the interface.7,36,37 However, other research found no difference between the Infant Flow Driver system and CPAP with prongs attached to a time-cycled, pressure-limited ventilator.38 There is limited clinical research on the RAM system, but analysis of the data showed that the sizes we used did not meet International Standard Organization requirements for flow resistance.39 These results were consistent with bench data, which found significant dampening of pressure transmitted to the circuit and test lung when comparing the RAM system with other cleared interfaces.13–15 This research indicated that pressure transmission to the lung with the RAM system during NIV and/or CPAP may be lower than those by approved interfaces,13–15 less than displayed circuit pressure, and highly dependent on percent naris occlusion, percent leak, and ventilator settings. We aimed to provide the high end of manufacturer's recommended naris occlusion (∼80%) in infants placed on the RAM system. However, our flows during CPAP and NIV surpassed acceptable mechanical flow resistance, although our settings were significantly higher after RAM implementation than those used in the control subjects. These changes may have compensated for the potentially lower transmitted pressures provided by the RAM system compared with the conventional interface.
There are many studies that detail nasal injury with noninvasive respiratory support in preterm infants.9,10,40–42 Several studies,9,10,40 including two that reviewed the Infant Flow Driver system,9,10 found the development of nasal trauma, even with the use of device rotation.9 However, two recent randomized controlled trials found a reduced incidence of skin breakdown when HFNC was used versus CPAP and/or NIV with prongs or masks.41,42 Our significant between-group results for any-grade pressure ulcer, therefore, were not surprising, although we attached the Infant Flow Driver system per manufacturer's instructions, then supported the circuit to decrease torque to the nose and tubing weight on the forehead. There usually was no need to provide pressure relief with the RAM system, however, we used it for extended periods of time in our extremely low birthweight population, which explains the occurrence of any lesion with this interface. When contrasting our results with the Nzegwu et al16 study, which found no new lesions with the use of the RAM system for < 10 d in low birthweight (1.29 ± 0.8 g) infants, our subjects on the RAM system were treated for an average of 37.9 ± 12 d, and weighed less (884 [range 497–1365] g).
The significantly higher ROP incidence in the RAM group was unexpected because we did not change our ranges for SpO2 after implementation of the RAM system. Between-group ROP-severity results, however, were reversed and trended with higher grades in the Control group (P = .059). Our target saturation ranges during both study periods were unchanged. Unfortunately, however, we do not have data on actual SpO2 values or delivered FIO2 for either study period, nor did we record data on the affected zones. In practice, it can be difficult to maintain appropriate saturations, with one study43 in preterm infants (N = 45) on CPAP finding SpO2 values within prescribed ranges only 31–39% of the total recording time (4,034 h). Anecdotally, we noticed higher nursing staff turnover after RAM implementation, and these newer associates tended to increase FIO2 when clinical status declined in infants on mechanical ventilation, with a failure to reduce the setting after resolution of the event.
Limitations
There were several limitations to our research. First, this was a single-center, observational study with a historical control group. Therefore, results may not be generalized and the potential for error in data extraction exists, which would bias our results. The infants were not randomized, and our total sample size was small. Therefore, randomized research with more subjects would be useful to further explore differences between the RAM system used with a pediatric ventilator and other systems. In addition, we did not account for multiparity or intra-uterine growth restriction, nor did we collect clinical information that might have been useful in interpreting some of our findings, such as additional ventilator settings, blood gases, or SpO2 values. However, resuscitation approaches and protocols related to mechanical ventilation did not differ between the study periods. Also, we did not have information on the frequency of nasal suctioning in this cohort, nor did we use any validated scoring system to grade the degree of skin breakdown. Therefore, our assessments did not include a severity grade but were based on choices available through our electronic medical record. Our staff was trained in pressure-ulcer identification, and these results were consistent with other findings in infants provided with noninvasive respiratory support. More research with skin-assessment scores to evaluate outcomes after noninvasive respiratory support in this population would be useful.
Conclusions
The ability to apply continuous distending pressure through the consistent application of noninvasive respiratory support with the RAM cannula attached to a ventilator may improve outcomes, including the duration of respiratory support and tissue breakdown, in low birthweight infants with respiratory distress syndrome. Although we found that the use of the RAM system corresponded with lower odds of developing bronchopulmonary dysplasia, the discriminate ability of the model was poor. The substitution of conventional noninvasive respiratory support systems with the RAM attached to a ventilator seemed to be relatively safe, but the rise in ROP rates we found should be further investigated.
Acknowledgments
The authors thank Edward A Palmer MBA RRT and Joseph P Lynott MSc RRT for their support in conducting this research.
Footnotes
- Correspondence: Gail S Drescher MA RRT, Pulmonary Services Department, MedStar Washington Hospital Center, 110 Irving Street, Washington, DC, BB42 (BH30). E-mail: gail.s.drescher{at}medstar.net.
Ms Drescher discloses relationships with SenTec and Neotech. Ms Hughes discloses a relationship with Neotech.
Supplementary material related to this paper is available at http://www.rcjournal.com.
See the Related Editorial on Page 1314
- Copyright © 2018 by Daedalus Enterprises