Abstract
Over the years a great deal of effort has been made to standardize all pulmonary function tests on adults. Many of the “rules” concerning the interpretation of the spirogram have been based entirely on adult observations. In the age of increasing conformity, and attempts to relate “adult” literature to the pediatric population, the latter was given much less emphasis than the former. This review will attempt to show what areas in pulmonary function testing are similar in adults and children, but more specifically will show the areas that are different. The latest standards published by the American Thoracic Society/European Respiratory Society in 2005 have attempted to incorporate some differences for the pediatric population for spirometry, but more work needs to be done in this area. While it is recognized that spirometry is the primary pulmonary function test for children, there are a number of circumstances where the addition of plethysmography and lung diffusion measurements are necessary. The review will state some of the limitations of these tests when performed by children. Lung function testing, particularly spirometry, has much to offer in the diagnosis of lung disease in children and the monitoring of response to therapy. With better standardization of pulmonary function testing in children, and more trained technologists, the age limits for testing can be extended to below 6 years of age and sometimes below 5. Also with better standardization the results obtained are meaningful and when interpreted in context of age offer excellent diagnostic information to better treat the child with lung disease.
Introduction
Pulmonary function testing began mainly in adults, as a means to diagnose and monitor progress of chronic obstructive pulmonary disease (COPD), which was becoming more prevalent due to smoking and occupational exposure. Some sort of volume measuring device has been around for a long time, but it was in 1866 that the water seal spirometer was connected to a kymograph so that both volume and time could be recorded. This concept was developed into the spirogram: the plot of expiratory volume during a maximal forced expiratory maneuver after a maximal inspiration against time. This has been followed by work done to define the correct way to perform spirometry, which includes the appropriate forced expiratory time (FET) and the concept of a “plateau” that indicates that expiration is complete1 (Fig. 1). Inspection of both the volume-time curve and the flow-volume curve demonstrates a good start, good effort, and complete exhalation.
Figs A and B represent acceptable spirometry. A shows the volume-time plot, indicating that a plateau was reached well before the 6-second forced expiratory time (FET) was reached. B shows the flow-volume curve, indicating a good start with a high peak flow. The flow gradually falls as volume falls. The measurements obtained are labeled in each figure. PEF = peak expiratory flow. (Courtesy of the Ontario Lung Association.)
Over the years a great deal of effort has been made to standardize all pulmonary function tests on adults. At present, many of the “rules” concerning the interpretation of the spirogram have been based entirely on adult observations. In the age of increasing conformity, and attempts to relate “adult” literature to the pediatric population, the latter was given much less emphasis than the former. With the development of pediatricians interested in lung disease of children, some of the tests and the physiology of “adult” lung diseases were “borrowed” from our “adult” colleagues and brought into the emerging sub-specialty of pediatric respiratory medicine. The latest standards have been published by the American Thoracic Society/European Respiratory Society (ATS/ERS) in 2005.1 In these standards an attempt has finally been made to incorporate differences for the pediatric population for spirometry. While it is recognized that there are differences, the trend of trying to adapt adult literature to pediatric literature has persisted. More work needs to be done for other routine pulmonary function tests such as diffusing capacity (DLCO) of the lung and plethysmography, interpretation of response to bronchodilators,2 and procedures for methacholine challenge testing.
This review will attempt to show what areas in pulmonary function testing are similar in adults and children, but more specifically will show the areas that are different. Some of these differences have been addressed in the ATS/ERS3 guidelines but some have not. The review begins with a discussion on measurements issues as well as performance and coaching issues. This review would not be complete without a discussion on differences related to reference values and interpretation of the pulmonary function tests. It is acknowledged that spirometry is the primary pulmonary function test for children, but there are a number of circumstances where the addition of plethysmography and lung diffusion measurements are necessary. The review will state some of the limitations of these tests when performed by children.
General Considerations for Testing
Environment
It is helpful for the area where testing is taking place to have a bright and pleasant atmosphere (ie, pictures on the wall, children's art and drawings). Having toys and books on hand is also useful if the child needs to wait a few minutes before testing and while waiting 10–15 min for post-bronchodilator testing.
Equipment
Mouthpiece.
There is usually no need to use a mouthpiece in addition to the filter. Children are usually very good about keeping their lips closed when reminded. A child size mouthpiece should be used if a better seal is needed, or for those patients with cranio-facial abnormalities for whom maintaining a seal is difficult.
Nose Clips.
Children do not generally have problems with the nose clips. When nose clips are applied, the children automatically start breathing through their mouths, unlike adults, who have to be reminded! Using nose clips with “spongy” tips stay on better on the child's small nose and are not so tight on their delicate noses. Also these types of nose clips are available in different colors. Letting the child choose the color he/she wants makes them feel a part of the testing. Of course some children will not allow application of the nose clip. Sometimes they may allow one of their parents to pinch their noses. As a last resort the spirometry can be done without the nose clips, but one should try to introduce them as testing proceeds.
Chairs.
Chairs should be adjustable to allow a small child to get into the chair. Ideally the child should be able to sit straight with both feet on the floor.
Measurement Issues
Expiratory Flow Rates
FEV1 Versus FEV0.75 Versus FEF25–75.
Since 1967,4 it was appreciated that forced expiratory flow (FEF) depended heavily on the static elastic recoil of the lungs. It is well known that children have higher elastic recoil and that the elastic recoil decreases with age. Various studies had demonstrated that the FEV1 correlated well with the degree of disability from lung disease. The impaired expiration in COPD is caused in part by a loss of elastic recoil that comes with the destruction of the pulmonary parenchyma and hyperinflation. Early pioneers in pediatric lung disease realized that healthy children had higher elastic recoil than healthy adults, with faster emptying of the lung, which meant that, as an indication of early lung disease, the FEV1 was relatively insensitive. Hence the early classic text on lung function measurement in children5 included reference values for the forced expiratory volume in three quarters of a second (FEV0.75) although, mainly in the interest of conformity to the adult literature, the FEV0.75 was largely supplanted by the FEV1, despite the recognition that, especially in pediatrics but also in adults, the latter was very insensitive to early disease.
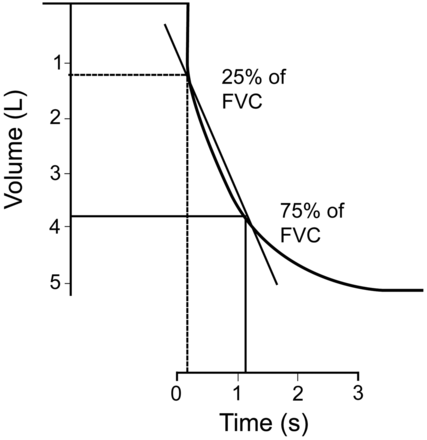
There was a search for more sensitive tests, capable of detection of disease in the small airways, believed in both COPD and cystic fibrosis (CF) to be the first affected. This led to a lot of interest in the measurement of flows at specific lung volumes, although when done by measuring volume at the mouth instead of in a plethysmograph as originally described,4 some of the physiology supporting this was later questioned.6 Despite a great deal of early enthusiasm for extracting data from the flow-volume curve, at present, taking numeric values from a flow-volume curve is not recommended2 and, in spirometry, numeric values should be taken only from the time-volume curve. While there are few tests specific for small airway disease in children, the slope of the points joining the volume-time curve at 25% and 75% of the volume axis (forced expiratory flow rates between 25% and 75% of the FVC, or FEF25–75) (Fig. 2)7 is believed to be the best indicator of early small airway dysfunction in children, CF being the classical example.8 It has also been shown to be a more sensitive indicator of disease in asthma than the FEV1.9
This figure is taken from an early water seal spirometer and shows the calculation of the forced expiratory flow during the middle half (FEF25–75) of the FVC maneuver. A straight line is drawn between the points on the curve at 25% and 75% of the vital capacity, and the change in volume with respect to time (slope) is the FEF25–75. (Adapted from Reference 7, with permission.)
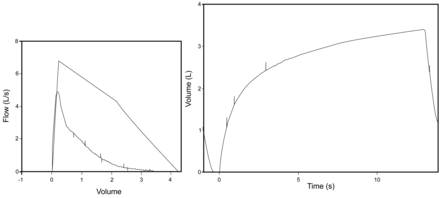
There was a brief period of popularity for the FEF25–75 in the adult literature, but this rapidly waned when it was realized that the coefficient of variation of the test, particularly in those with COPD, was excessive. This is primarily due to the fact that the calculation of FEF25–75 is dependent on the FVC. In adults with COPD, as well as children with moderate to severe air-flow limitation, expiration may not be complete at the end of the minimally recommended 6-second expiration, and a plateau may not be achieved (Fig. 3) even after 12 seconds of expiration (recommended stop of test). For some patients, the longer they attempt to exhale, the greater the volume or the FVC. This means that in many cases the FVC depends in part on the FET which, after the minimal 6 seconds in adults and 3 seconds in small children, is not standardized. Since the FEF25–75 is the slope of the line between the 25th and 75th percent of the FVC, any variation in the FVC makes a substantial variation in the FEF25–75, resulting in the excessive coefficient of variation. For children and young adults with greater elastic recoil, the variability of the FVC with FET is less, and hence the coefficient of variation is much less. However, this does give rise to some interesting physiology. In early CF, when the disease is largely confined to the small airways in the periphery of the lung, the FEF25–75 is an excellent way to follow the disease. However, with the progressive loss of elastic recoil with increasing disease, the FEF25–75 tends to “bottom out” at less than 20% predicted and becomes dependent more on FET and hence unreliable. In these situations the FEV1 becomes a better way to follow disease. Since one of the major values of the FEV1 is the correlation with disability, regardless of whether or not the disability is from restrictive or obstructive disease, the question arises if this is true in pediatrics. When the point at which the risk of lung transplant exceeded the risk of dying from CF was considered,10 the cutoff of 30% predicted in the FEV1 was proposed. This has been challenged with more sophisticated approaches, but to date has stood the test of time.
This figure is an example of a 14-year-old child with obstructive lung disease due to cystic fibrosis. The forced expiratory time (FET) is greater than 12 seconds, and yet a plateau is never reached. In this case the FVC will depend on the FET. Since the repeatability of forced expiratory flow during the middle half (FEF25–75) of the FVC maneuver is critically dependent on repeatability of the FVC, changes in the FET after the mandatory 6 seconds will give rise to substantial variability in the FEF25–75.
FEV1/FVC
The index that has received much attention in adults but only sporadic attention in pediatrics is the ratio of the FEV1/FVC.9 One of the challenges in the past with this has been the recognition that the parameter changed with age, and there was a paucity of normal reference values. This should no longer be the case.11 One caveat is that it is essential that we have a full inspiration and expiration. It is often possible to get accurate FEV1 values with children, but not so with FVC, since younger children may have difficulty understanding blowing out to residual volume (RV).
The higher elastic recoil and more rapid emptying of the lungs in the pediatric population can also lead to a higher FEV1/FVC ratio, which in adults could be interpreted as a restrictive pattern, but can be considered normal in children.12
Performance Acceptability Issues
Back Extrapolated Volume
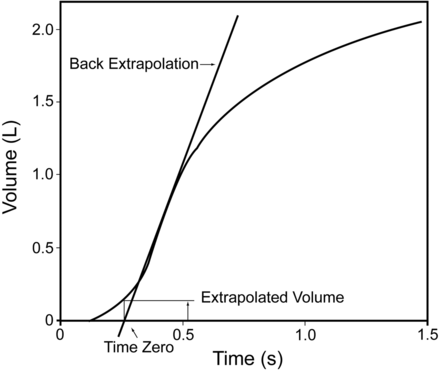
The “back extrapolation volume” (BEV)1 is a measure of how fast a start there was to the forced expiration. It is absolutely essential to have an accurate time zero for any time related volume. Since no physical action starts “instantly,” the part where a line with the steepest slope on the volume-time curve crosses the zero volume on the time axis defines time “zero.” (Fig. 4). In the presence of a slow or hesitant start, the volume at zero on the time axis will be high. The current ATS/ERS recommendation is that if the volume is greater than 5% of the FVC or 150 mL (whichever is greater), the effort is not acceptable. This recommendation does not distinguish between adults and children, which causes substantial problems in small children when the technologist must decide on whether or not the effort is acceptable. It has been proposed13 that in children this value be changed to 80 mL or a maximum of 12.5% of the FVC. Unfortunately this recommendation came too late to be incorporated into the 2005 ATS/ERS standards. Therefore the computer software for spirometry testing does not recognize any differences in the BEV between adults and children.
This figure demonstrates the method for calculating the time zero with the back extrapolation. In this case, in an older child with an FEV1 well over 1.5 L, the extrapolated volume is relatively small and within the 150 mL limit. (Courtesy of the Ontario Lung Association.)
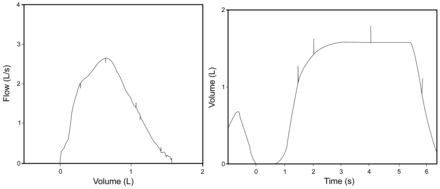
Consider then a child with a 1 L FVC and a BEV of 149 mL. That child's effort will be considered, by the computer, to be acceptable, as the BEV falls under the 150 mL exclusion criteria, but in fact represents 15% of the FVC and should in no way be considered acceptable (Fig. 5). Current standards insist that the computerized display include both the volume-time curve and the flow-volume curve and that these be scaled appropriately to allow visual inspection. Technologists who are accustomed to visually inspecting curves of children will recognize that, while technically meeting the software and ATS/ERS criteria for acceptability, the small child could have done much better and they will not accept efforts with slow starts.
This figure shows an example of a test with a hesitant/slow start, where the back extrapolated volume of 0.14 L is not detected as an error, despite the fact that it amounts to nearly 10% of the FVC.
Inspiration Time
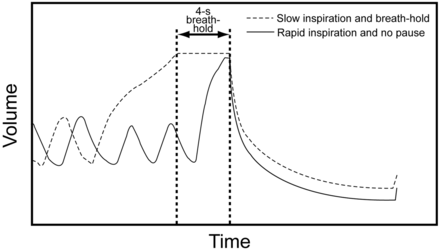
Another challenge in spirometry is to recognize when the patient has inspired fully and to have them blow out with little hesitation. The standards say that the inspiration should be rapid and there should be no breath-hold at end inspiration,1 but knowing that the child has reached end inspiration can be challenging, and often the child is encouraged to effectively breath-hold at total lung capacity. The problem with this is that the resulting FVC and FEV1 is less after a slow inspiration and 4-second breath-hold in the presence of any important disease such as CF14 (Fig. 6).
This figure is adapted from a publication14 where children and young adults with cystic fibrosis were asked to perform spirometry with no pause after a rapid inhalation to total lung capacity (solid line) or to take a slow inhalation and a 4-second breath-hold at total lung capacity (dotted line). With the slow inhalation and breath-hold, both the FEV1 and FVC were significantly lower than if there was no breath-hold. (Adapted from Reference 14, with permission.)
Minimal Expiration Times Criteria
One of the ends of test criteria is that the expiratory effort must be sustained for at least 6 seconds and then a “plateau” must be achieved (ie, no change in volume [< 0.025 L] for at least one second). The 6-second criteria was established to ensure that an accurate FVC be obtained, as other measurements, the FEF25–75 and FEV1/FVC, as well as total lung capacity and RV, and to a lesser extent DLCO, are dependent on a maximal inspiratory and expiratory volume maneuver. This is easily achieved in normal adults, and in fact young healthy adults can completely expire in 2 to 3 seconds. Elderly adults and those with obstructive lung disease may take longer than 6 seconds to empty their lungs and achieve a plateau, while those with COPD never obtain a true plateau. Adults with restrictive lung disease will have trouble achieving the 6-second criteria, due to shortness of breath, and they empty their lungs very quickly, often very close to 1 second.
This is similar to a healthy child. They have low normal lung volumes and high elastic recoil. Many efforts were rejected as not acceptable in children because they could not sustain the effort for 6 seconds. This has now been changed, and the end of test criteria for children under the age of 10 is 3 seconds. The choice of the cut-off age of 10 years is interesting. If the technologist is able to make the child understand to blow out to RV and sustain this effort, most 7-year-olds can exhale for 6 seconds, although a plateau was achieved much earlier. On the other hand, some 11-year-olds may have problems with the 6-second expiration, but, again, achieve a plateau much earlier. One can argue as to why there is such a minimum time criteria. The end of test criteria could simply be the achievement of a plateau (no flow for at least one second), regardless of age or disease.
Repeatability Criteria
In order to increase the number of “technically acceptable” tests, the criteria for repeatability, which previously depended on serial tests for FVC and FEV1 to be within 5% percent15 of each other, was redefined to an absolute volume in order to improve acceptability that appeared to be related mainly to subject size.16 This has been further refined1 to suggest that the criteria for repeatability be measurements for FEV1 and FVC within 150 mL when the measurement is greater than 1 L, and 100 mL if less than that. Since the original goal was tests within 5% of each other, for a child with an FVC of just over 1 L, the criteria allows a 15% variation.
With the official criteria for repeatability being relatively so much larger for small children than adults, often not as much effort is made to see if there can be improvement over a 149 mL difference in measurements just over 1 L. In pediatric pulmonary function laboratories with experienced technologists, it is recognized that the actual repeatability performance is very much better than these limits. This can actually be the case in adult labs as well.
Bronchodilator Administration and Reversibility
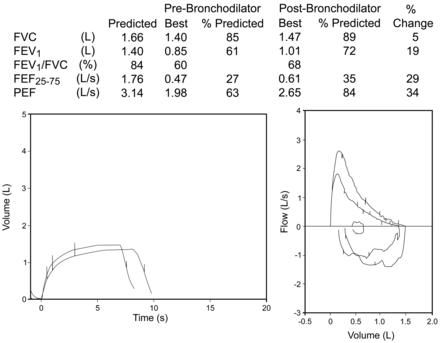
It is recommended that 4 separate doses (each 100 μg) of albuterol/salbutamol be delivered when assessing bronchodilator reversibility. This is to ensure the dose is sufficient to evoke a change if one is going to occur.1 A lower dose can be used if there are concerns about the effect the dose will have on the patient's heart rate or tremor. There are no indications that this should be different for children. The new ATS/ERS standards for interpretation2 suggest a minimal change for significance is a 12% increase in FEV1 or FVC, with a minimal absolute change of 200 mL. There is no reference to children; 200 mL represents a much larger relative volume in a small child than in an adult (Fig. 7). For a child with an FEV1 of 1.0 L there would need to be a 20% increase (200 mL) for it to be considered important. The latest health survey in the United States, the National Health and Nutrition Examination Survey IV (NHANES IV), does include bronchodilator assessment and may add substantially to the pediatric literature when completed.
This figure shows that the child improved by 19%, but this is not even 200 mL, and thus would not be considered a positive result as per the American Thoracic Society/European Respiratory Society “adult” criteria, and illustrates the need for pediatric specific criteria.
The choice of the minimal requirement is to be reasonably sure that the change is well outside the normal coefficient of variations of the measurement, in other words, highly unlikely to have occurred by chance alone. This is a statistical decision, not a physiological one. For example, if a child was tested 10 times and each time had a response to bronchodilators, of 10%, the possibility that this occurred by chance and chance alone is vanishingly small. The bottom line here is that in all subjects, but particularly in children, the interpretation as to whether or not a response to bronchodilators is important or not is a judgment call, with no absolute rules to follow. Furthermore, the concept that a response represents the diagnosis of asthma with no other supporting information is simply not valid nor is the opposite, in other words, a lack of response rules out asthma.
Coaching Differences and Tips
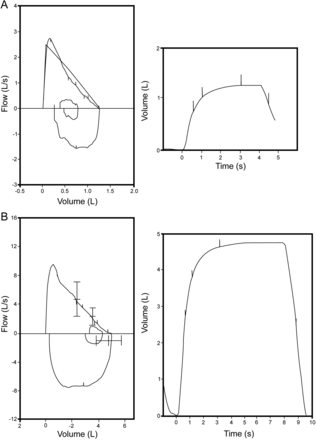
There can be some technical differences between adults and children when performing spirometry, and these differences must be taken into account, but ultimately what we are trying to obtain from a child is the same as an adult. See Figure 8. Can you determine which curve is the child's and which curve is the adult's? In both cases the patient took a big breath in, blew out hard and fast, and blew out to RV. The only difference between the curves is the size (ie, a 5 L capacity versus a 1.25 L capacity). How these curves were obtained could have been also very different.
A is a spirometry effort by a 5-year-old child, and B is that of an adult. How can you tell? Both meet the acceptability criteria. The difference is the actual vital capacity, 1.25 L for the child and 5 L for the adult. Both established a plateau, within 3 seconds for the child and 6 seconds for the adult. Note that the volume axis has been scaled to size, as recommended (American Thoracic Society/European Respiratory Society). This is not an option in all spirometers.
Technologists working with adults learn various coaching techniques to obtain an acceptable curve. Different coaching techniques are needed with children. Instructions must be kept very simple. Demonstration of the maneuver is imperative. Gentle encouragement is needed and intimidation avoided. The child must be kept focused on the task at hand.
Start of Test Criteria.
Usually if the patient understands the “blast/blow out hard,” the BEV is not a problem. The patients must understand that “the blow out hard” comes from using the abdominal/costal muscles to achieve peak flows. Often with adults the technologist will forcefully say “BLOW!” and even stomp his or her foot. This may not be a very good idea with the young children. Frightening them may not work. Verbal cues such as “blast it out like someone has punched you in the stomach” are often very effective in children.
End of Test Criteria.
Blowing out to RV and sustaining that blow out is a difficult concept for the young children to understand. The verbal instruction of trying to blow out all of mommy's candles on her birthday cake because mommy is really old and has more candles than you is something that children readily understand (and enjoy). All children know that you are supposed to blow out all the candles in one breath.
Incentive Spirometry.
Visual feedback is also another coaching tool. The standard spirometry program on the computer screen can be helpful. The program inserts the 6-second line, and thus the patient can be instructed to keep blowing until they get to that line. Also showing the children how they can create “mountains.” Using the predicted curve as their goal to beat the computer works very well. Some programs have very innovative incentive programs. The child can visually see the flickering candles or the bowling ball heading down the alley to knock over the pins. The point of the incentive spirometry is to “blow out long.” Unfortunately, when a child uses the incentive spirometry, he/she often loses sight of the other criteria (ie, big breath in and blow out hard). They no longer listen to the technologist, and they begin to play games with the computer.
Reference Values and Interpretation
Reference Values
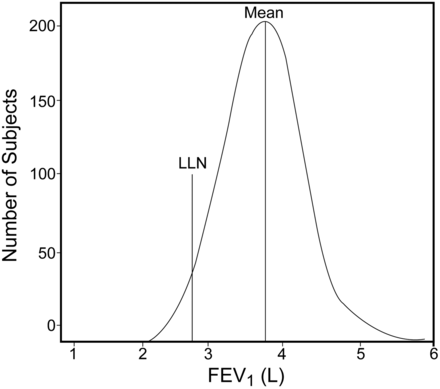
One of the new areas of controversy facing both the adult and pediatric pulmonary function world has to do with interpretation. Historically, most interpretation has been done on percent of predicted, whereas in most other areas of laboratory medicine, the lower limit of normal (LLN) is used. Until recently, using the LLN for pulmonary function testing has been hampered by the relatively small number used to generate the regression equations, which meant a lack of statistical confidence in the LLN. This is no longer the case with the NHANES III,17 which, from the pediatric perspective, was limited in that it did not deal with children younger than 8 years. The age issue has been addressed recently by Stanojevic and colleagues,11 where other small pediatric series were combined with the NHANES III data to extend the lower age limit. The LLN was defined as the lower 5% of the population in relation to age, sex, and ethnicity, ranging from 5 to 80 years of age. This corresponds to a “z-score” of −1.64 (Fig. 9). There are 2 main limitations with the Stanojevic data. First, they pertained only to whites, and, second, the statistical analysis gave rise to equations that are so complex that it has proved challenging to add to the software of commercial systems.
This is a schematic representation of the distribution of the FEV1 with the lower limit of normal (LLN) (the short vertical line) at −1.64 standard deviations from the mean. This represents the lower 5% of the population. The long vertical line is both the median and the mean, since the values are evenly distributed around the mean. (Courtesy of the Ontario Lung Association.)
The huge advantage of using the LLN is that it takes into consideration the changes in the predicted value over age. In other words, interpreting the FEV1/FVC as an index of abnormality would be based on the patient's age. While this may be more of an “adult” issue in that the predicted value FEV1/FVC changes dramatically from mid-twenties to the elderly, it is still a pediatric issue, as there is a change in elastic recoil of the lung and respiratory system between early childhood and the late teenage years.12
Interpretations
Determining the presence of disease can sometimes be difficult in the adult population. Using adult criteria to interpret a child's pulmonary function tests is cause for concern. A further issue is that the definitions of the degree of severity in the new ATS/ERS standards for interpretation2 are all based on percent of predicted, and, more disturbing from the pediatric point of view, based entirely on adult data. Ironically, the data to support the choices of degree of severity in lung disease in adults was largely based on outcome when the FEV1 was analyzed expressed as a z-score.2 Inherent in the table outlining severity2 is the assumption that the LLN, the z-score of −1.64, will not change with age, although reference equations make it clear that it does. Interpretation of the pulmonary function testing is particularly challenging in children. Care must be taken when choosing a “parameter” to monitor disease and response to therapy in the child versus adult. One must recognize that there are limitations to the other tests, and they do not often provide more information.
Other Routine Pulmonary Function Tests
Other “adult” pulmonary function tests have been used widely in children, and these include lung volume measurements by plethysmography and DLCO. While both measurements have important application in a tertiary care pediatric setting, for the vast majority of children with lung disease these tests will not add much to what has been indicated by spirometry. Obvious exceptions to this would be children with restrictive lung disease, where lung volume measurements would be important, or children undergoing chemotherapy with potentially lung toxic agents such as bleomycin, where serial unchanging measurement of DLCO provides some assurance that lung toxicity should not limit chemotherapy.
Plethysmography
There are no technical limitations to performing the FRC measurement in the body box. In fact, children do much better with this measurement than adults. They automatically continue panting when the shutter closes and do not suffer from claustrophobia in the degree that adults do. Therefore, if the child is able to perform spirometry adequately, plethysmography will then be very useful in identifying restrictive disease.
DLCO Minimum Requirements
In earlier days, the collection of gas for the measurement of DLCO was an actual sample collected in a bag. The collection was made after it was assumed that the predetermined dead space had been washed out, usually 750 mL. Advances in computer technology and rapid-response multi-gas analyzers provide real time analysis and a “virtual sample volume.” This allows retrospective adjustments of the sample volume based on the actual dead space washout determined by inspection of the concentration of the tracer gas. These potential operator adjustments have allowed more accurate measurements of DLCO in children and individuals with small lung volumes.
Current ATS/ERS guidelines state that the washout volumes can be reduced to as low as 0.5 L in patients with lung volumes smaller than 2 L, while trying to maintain a sample volume between 750 mL and 1 L. In patients with a VC less than 1 L, a sample volume of 500 mL can be used once ensuring dead space volume has been cleared. In reality, it is difficult to obtain a DLCO from patients with a VC lower than 1.5 L, especially in those with poor gas mixing, which increases dead space, while still trying to maintain accurate and reproducible results.
Reference Values
For spirometry large series of reference values exist. This is simply not the case with DLCO in white children, and even more of a problem in children of other ethnicities. The choice of reference equations for plethysmography is also limited18 and restricted to whites.
While, as stated above, it would often be very useful to obtain more information on a child's lung function, one must recognize the limitations when the child is unable to perform spirometry correctly. While the child may be able to perform the FRC plethysmography “panting” maneuver and the DLCO “breath-hold” adequately, the inability to perform a maximum vital capacity will render the results of these tests grossly inaccurate, leading to incorrect interpretation, and therefore proving more of a hindrance than a help.
Tests for Those Too Young to Cooperate With Standard Spirometry
The above discussion was about children who have the ability to understand how to perform spirometry if coached properly, and whose limitations are understood by the technologist. Typically a child around 5 years old will have a 50% chance of performing spirometry adequately in a child orientated lab with technologists experienced in pediatric testing, but chances may be much less in an adult orientated environment. It is very unlikely to obtain full spirometry from a younger child.
More recently there has been much interest in lung function measurement in children normally considered to be too young to cooperate with standard spirometry. Tests have been proposed to evaluate lung disease in infants ranging from small preterm infants on a ventilator,19 toddlers from 1 to 2 years of age (infant pulmonary function),20 to attempts to have preschool children perform a variation of spirometry.12 There is a recent excellent review of a host of techniques,21 and a detailed description is beyond the scope of this review.
Summary
Lung function testing, particularly spirometry, has much to offer in the diagnosis of lung disease in children and the monitoring of response to therapy. There has been much progress over the past decade or so regarding the standardization of spirometry, which includes differences between children and adults.1 While the ultimate goal of pulmonary function testing is the same for children as for adults, and instructions and graphs (flow-volume curve and volume-time) are similar, there are many differences that must be understood to accurately assess lung function in a child.
The various measurements obtained from spirometry to monitor disease and response to therapy must be evaluated in the context of age and presence of lung disease at the various ages. Some differences in performance of maneuver and acceptability criteria have been identified, but more work needs to be done in this area. Changes such as lowering the acceptable FET for younger children to 3 seconds, and the lowering of the standards for repeatability criteria for FVC and FEV1 from 150 mL to 100 mL for those with values less than 1 L, are all a step in the right direction. However, the use of adult guidelines in the assessment of factors such as BEV, repeatability, and bronchodilator responses permits “allowable” errors into pediatric testing and interpretation that would not be deemed acceptable within adult testing.
The technologist must change coaching techniques when working with children in order to obtain good pulmonary function results. Interpretation of the pulmonary function testing remains particularly challenging in children. Normative reference values for spirometry are good, but much work still needs to be done, especially for normative data on plethysmography and DLCO.
Newer equipment and trained technologists are much more “child friendly” than in the past, which extends the age limits for testing, often below 6 years of age and sometimes below 5. With this age group it must be recognized that while useful information can be obtained from spirometry not properly performed, there is no benefit to having this child continue testing for plethysmography and DLCO. For children too young to cooperate with standard spirometry, there are a variety of other options, although, at present, few have found their way into busy clinical laboratories.
Discussion
Miller:
So much of what you said about spirometry in kids applies to grownups as well. I'd like to ask 2 questions. The spirometers that only measure expiration and only display expiration, where you take your full inspiration and then manage to get your mouth around the mouthpiece: should they be banned? Because there is no way to assure that we're dealing with full inspiration.
Coates:
I'm reluctant to use the word banned.
Miller:
OK, well, discouraged. Strongly discouraged.
Coates:
Yes, for a couple of reasons. One reason is the inspiratory history is important, you should see the subject inspire before expiration. You have no idea about the inspiratory history when you have those spirometers. For children, they are very hard to use, and for the smallest children, getting the mouth piece into their mouth while holding their breath and then go all out to residual volume is not the easiest. I do not think that they address the pediatric needs, and they may not address adult needs either.
Miller:
They certainly don't address geriatric needs, because an 82-year-old non-English-speaking fragile nursing home patient is no more capable of that. My second question is a totally different one. You spoke about spirometry. Is there a role for measuring especially diffusing capacity and then lung volumes in kids beginning at a certain age? And which age?
Coates:
Children have more difficulty with plethysmography than they do with spirometry. In our CF clinic we start with spirometry, and we'll probably start when they're 5, and usually get good results when they're 6. We start to get decent plethysmographic values when they're about 8. I actually had to have a debate about spirometry versus lung volume measurements, and I lost horribly, because the person who was on the other side pointed out very clearly (and I don't disagree with him) that, in the vast majority of circumstances, spirometry probably tells you all you need to know. However, there's no question that when you're dealing with complex diseases, lung volumes are invaluable. I didn't get into lung volumes, partly in the interest of time, and the other issue that Neil [MacIntyre] raised earlier, which is certainly our situation as well, is that our oncologists are very fond of using DLCO to look for bleomycin toxicity. We tend to do DLCO on any patient where we suspect there may be some reason, especially something that might be reversible, so, yes, we do lung volumes and DLCO.
MacIntyre:
Your third bullet point on that slide, I always find it interesting, because you could say the same thing about adults. I'm not sure what to do with these numbers, if you have an abnormal test, no matter how bad it is, it usually means that there's a higher risk of surgery and post-operative complications. But, at least in adults, there are no absolute cutoffs. It's not like you might not do surgery if their FEV1 is above 1 L, but not if it's less than 1 L or something like that. Are you any better in kids with coming up with threshold values? Or are you, like us, where you comment on the increased risk and leave it at that?
Coates:
We have a threshold value for back surgery, but the data on which these rest are very vague. Usually if the FVC is ≤15 mL/kg body weight, we can extubate post-operatively.
MacIntyre:
So you're like us in adults. I don't think we've ever turned anybody down for surgery based on PFT [pulmonary function testing]. Or, if it's happened, it's extremely rare. We usually just comment that it could be a stormy post-op course.
Coates:
The discussion we would have in our home-ventilation clinic is that after surgery we may not be able to get away with BPAP [bi-level positive airway pressure]: it may be tracheostomy and ventilation, so before we go into surgery, we want you to consider this as an option or whether the child wants to stay with his or her current condition and choose a more palliative approach. The parents and children vary in their response, but the cutoff is more of a warning value for us. Many children below our cutoff, who go through scoliosis surgery, get extubated and do fine. However, the only time I've seen children who have major post-operative respiratory complications, they are below our cutoff.
Pichurko:
I had the occasion to test 12-year-old swimmers, obviously at the peak of their respiratory health. One young athlete generated a spirometry record with an FVC value but a blank space with no entered value for FEV1 and FEV1/FVC. A very good percent predicted FVC was generated, so I felt confident that the expiratory effort was complete. Do you see that in the general pediatric population?
Coates:
I'm not sure, with no signal. Even if the FEV1 and FVC are the same, if there is a long enough effort, the test will be valid and there should be a print out.
Pichurko:
The young athlete expelled the entire FVC in less than one second, so the test was complete with no recorded signal at one second. As I only had the experience with very athletic young people, I wasn't sure whether such excellent airway function was also seen in the general healthy pediatric, but not specifically athletic subjects.
Coates:
Many children with neuromuscular disease have such stiff chest walls that by half a second they're at FVC.
Kaminsky:
I wanted to make the educational point again, that applies equally as well to kids as adults, that is when we teach our residents and fellows about lung function testing with all the computerized equipment we have and so on, it's essential that we go back to basics and show them curves and how to measure slopes and other details. Otherwise, there's no way to troubleshoot what's behind the scenes.
Coates:
I couldn't agree more. There's one other thing about the fellows; our fellows believe that the people who wrote the ATS/ERS standards1–3 went to the mountain and they came back with the stone that was carved from above. I point out that I was actually at many of those meetings; if they happened in North America, the Europeans were jet-lagged; if they happened in Stockholm, the North Americans were jet-lagged, and even when not jet-lagged, there was a lot of controversy with almost every decision as to what would be the standard. In other words, the standards are nothing more than educated guesses by people who hopefully have had enough knowledge and experience to be credible. The fellows have to learn there are physiological disagreements, there are errors, and there are changes required. No guideline is perfect: every guideline is a compromise, and hopefully the newer guidelines address the problems with the old.
Culver:
Allan, I wanted to ask you a little more about end of test criteria and the drop-off that you were concerned about representing early termination. Earlier we talked about middle-age to older individuals having airways closure as their limitation, and they tend to asymptote down into the zero line. But the same study I got that from, which was a nifty study1 by David Leith and Jere Mead many years ago, where they did the bear hug equivalent that we talked about earlier at end-expiration. They found that young adults did tend to have a little bit of a drop-off, and they interpreted that as chest wall limitation, because additional air could be pushed out with an external squeeze. The airways were still open and air flow was slowing down but still going forward until the chest wall and diaphragm hit their limitations, and then a fairly abrupt drop-off was seen at the end of the curve. It made not a vertical drop, but it nosed over rather than the sloping ski-jump type that we're expecting. I would expect to also see that in little kids, but maybe you don't, they do have smaller airways and maybe they behave differently.
Coates:
Well, you do, except the difference between that and glottis closure is quite striking. It's usually glottic closure that is the premature termination. When they just peter out, you see it go more asymptotic, but when they push all the way, you see it come down fairly quickly. Yes, RV in young people without lung disease is determined by elastic recoil of the lung versus respiratory muscle strength. If you look at the pressure-volume curve, the pressure-volume curves for the chest wall become flat at RV, so pushing harder and harder doesn't get that much more out. Now, somebody coming up behind and squeezing will change that curve.
Salzman:
You were making a point about training for pulmonary function technologists, about the need for more specific skills for testing children. I'd really like to raise the general point—that I'd like the whole group to comment on—in some ways I'm seeing PFT technology as an orphan field in terms of training pathways. What I mean is, people who are not going to be physicians but want to work in the health care field have really good training pathways to become radiology technologists, ultrasound technologists, echocardiography techs, and of course in our field, respiratory therapy practitioners, but there are not great pathways for training as PFT technologists. Certainly the certification and registry pathway helps ensure quality, but if you all could comment on where might we go in the future to really keep the quality up in the PFT lab?
Coates:
CoARC [the Committee on Accreditation for Respiratory Care] is extremely active in developing certification programs, which I think is great. In Saskatchewan, Brian Graham developed a national working group that organized a training course for people who would be doing spirometry in primary care settings—the SpiroTrec course—which I also think is very good. Once we've got these courses developed, we then have to make sure that the people who are doing the testing take the course.
Bob Crapo kept saying, time and again, at the meetings, “you do all the training, and then if you watch the spirometry, month after month you see it deteriorate if there is no ongoing feedback.” So not only do you have to do the training, but you have to have some continuous ongoing quality assurance. I think this is a huge challenge in adults and in pediatrics. The part that's a little bit frightening, if I can editorialize a touch, I think there probably are drugs that have some value that have not gone through because of inadequate training, inadequate equipment has put so much noise in the signal that a truly detectable difference was not found.
So when I talk to people in the pharmaceutical industry they're planning a study and they say, “we'll use a multi-center study,” and I keep coming back to, you have to look at the quality of spirometry or whatever test you're going to use at those centers. This is particularly true if you're going to go center to center to center and do DLCO on different equipment. We do not have the standardization of DLCO equipment that we do for spirometry, the noise in that signal could be huge. I think that it's a very valid point; it's a point that causes a huge problem.
Ruppel:
Jeff Haynes will speak about credentialing of technologists and laboratories tomorrow. I think you're correct when you say that the credentialing of pulmonary function technologists leaves a lot to be desired. Most of the folks who take the NBRC [National Board for Respiratory Care] credentialing exams are trained in respiratory therapy programs, and, just like a lot of resident programs in pulmonary medicine, they get less and less hands-on experience with PFT. In many hospitals in the United States the RT department runs the PFT lab, so practitioners may be doing PFTs one day and then be in the intensive care unit, and may not be back in the lab for another month. All of those factors cause problems. In addition, the physicians who are many times interpreting the tests may have trained in a place where they didn't get a lot of hands-on experience. We try get them into the lab, to be sure they understand what an FEF25-75 is.
Coates:
Years ago when I was at Montreal Children's Hospital and we were starting a lung transplant program with McGill University, we decided we wanted to do the pre- and post-pulmonary function measurements in the same place, so we brought them down to Montreal Children's Hospital, where we had a technician who I'm sure could get an FVC out of a stone. She routinely got something like 600 mL more FVC than they had at the adult referral hospital. You can't just say, “Hi dear, take a big deep breath all the way in, now blow it out.” You could hear her do PFTs several offices away, and the patients came out a little shaken, but they had certainly produced a much greater FVC.
MacIntyre:
You bring up a point that you and I have talked about before. Maybe we're relying too much on tests that require a lot of patient cooperation like DLCO and FEV1. Maybe things like impedance oscillometry, where you just have to breathe—would that be a way to address some of this standardization issue?
Coates:
I published a couple things1,2 on impedance oscillometry, because we were very excited when it first came out, because we thought it would give us a handle on lung function measurements on children too young to cooperate with spirometry. The problem is that you have to be absolutely still, with your head and neck so they are in exactly the same position. What we found is that children who were too young to cooperate with spirometry were moving their heads, and as soon as you start changing your neck in little children, values start to change. Furthermore, a different head position from one day to the other may give rise to differences in values that have nothing to do with disease. So theory may be better than reality.
There are some people who are very enthusiastic about these tests and go to great lengths to make sure there is no movement of the head and neck, but as a routine, oscillometry in children too young to cooperate with spirometry did not do as well as we had hoped.
Miller:
A very simple question about reference values. In a number of labs that I've been familiar with, which primarily test adults and are doing the occasional kid, the fallback is always Polgar.1 Is this a reliable fallback across the board?
Coates:
Absolutely not, as it is well out of date.
Miller:
So what does one do in that kind of lab, which is encountering say, one kid a week?
Coates:
If children are more than 8, NHANES III is a very good set of reference values.1 If the children are less than 8, Stanojevic's new data2 are actually excellent reference equations, except that it's fairly complicated to use and you need software. To date, manufacturers have not been willing to install it, or haven't quite gotten there.
Miller:
And then what do you do if you do try to do diffusing capacity?
Coates:
Oh, we are using some data that was generated by Tom Keens years ago when he was a fellow. Now, he's my vintage. We're looking for new normal values and I was busy trying to persuade Statistics Canada to institute normal DLCO in their Canadian Health Surveys measure, and I would urge any of you who have influence with NHANES IV to do the same thing in the United States, because we're in desperate need of better reference values in adults and in children.
Rundell:*
I have a question. For the 6 and 7-year-olds, what would you recommend?
Coates:
The best one is Stanojevic,1 there's no question. You won't be too far off if you go with Corey and Levison,2 all of which went into making up the new Stanojevic equations. The Stanojevic equations are based on all the NHANES III data,3 and then it took a lot of data from other people, which overlapped the NHANES data in those over 8 years of age, but they had data going down to 5 years of age. Then they developed the equations, which were stable over the whole age range.
Footnotes
- Correspondence: Allan L Coates MDCM B Eng(Elect), Division of Respiratory Medicine, Hospital for Sick Children, 555 University Avenue, Toronto, Ontario, Canada M5G 1X8. E-mail: allan.coates{at}sickkids.ca.
Dr Coates presented a version of this paper at the 48th Respiratory Care Journal Conference, “Pulmonary Function Testing,” held March 25–27, 2011, in Tampa, Florida.
The authors have disclosed no conflicts of interest.
↵* Kenneth W Rundell PhD, Pharmaxis, Exton, Pennsylvania.
- Copyright © 2010 by Daedalus Enterprises Inc.