Abstract
BACKGROUND: Arterial blood sampling is subject to numerous pre-analytical errors, one of which is inadvertent venous blood sampling. Especially when assessing oxygenation and titrating ventilation, accidental venous blood sampling may lead to inappropriate respiratory care and repeated percutaneous punctures.
OBJECTIVE: To determine the effects of mean systemic blood pressure and needle size on vented arterial sampler filling times, to distinguish venous and arterial sampling.
METHODS: We constructed an extracorporeal laboratory model to circulate whole blood at 4 L/min. We used hemostats to create 6 pressures: 57 mm Hg (representing a patient in shock), 70 mm Hg (representing a patient with low-normal blood pressure), 93 mm Hg (normal), 107 mm Hg (high-normal), 133 mm Hg (severe hypertension), and 14 (peripheral venous pressure). We simulated percutaneous punctures with vented arterial samplers preset to 2 mL, with 2 common sampling needles. We compared the filling times of each pressure/needle combination and determined the correlation between the mean pressure and filling time.
RESULTS: For all the tested arterial pressures combined, the mean ± SD sampler filling time was 15.8 ± 0.4 s; for venous pressures the time was 51.4 ± 1.4 s (P < .001). With the 22-gauge/1.5-inch needles the sampler filling time was 22.2 ± 14.9 s. With the 23-gauge/1-inch needle the time was 21.4 ± 13.1 s (difference not significant). The Pearson correlation coefficient between the mean blood pressures and the sampler filling times was r2 = −0.86 (P = .01).
CONCLUSIONS: Lower blood pressure increased the sampler filling time. Measuring the filling time may enable therapists to confirm successful arterial puncture in adult patients. Confirming successful arterial puncture prior to blood analysis would expedite appropriate patient care decisions.
Introduction
The analysis of arterial blood gases is frequently used in intensive care units and various hospital settings to monitor oxygenation and ventilation in a broad spectrum of patients.1 The importance of performing arterial blood sampling and analysis competently and correctly is self-evident.2 Pre-analytical errors can affect the blood gas results and adversely affect patient care.3 One pre-analytical error is inadvertent venous blood sampling. Especially when assessing oxygenation and titrating ventilation, accidental venous blood sampling may lead to inappropriate respiratory care and repeated percutaneous punctures.4 Obtaining venous blood instead of arterial blood can be unrecognizable until the results are analyzed and correlated with the patient's clinical condition. Visually verifying arterial blood based on pulsatile blood return can be difficult, and based on blood color may be misleading in hypoxemic patients, whose blood may appear dark in color, similar to venous blood.5 The replacement of syringes and moveable plungers with vented arterial samplers has removed arterial blood pressure as a reliable arterial indicator.6 Because of the cost of arterial blood gas analysis, cost is another important reason to confirm that blood is arterial.
The purpose of this study was to determine if the time to fill a vented arterial sampler is dependent upon various mean arterial pressures and venous pressures, and on the size of the sampling needle. Arterial sampler filling time might provide a means of determining, at the time of sampling, whether the sample is indeed arterial blood.
Methods
This study was performed in the Respiratory Therapy Laboratory of the School of Allied Medical Professions at The Ohio State University, Columbus, Ohio. We conducted a laboratory experiment to evaluate the effects of 6 mean blood pressures and 2 common-size arterial sampling needles on the filling times of vented arterial samplers. By consulting textbooks of human physiology and pathology, we identified blood pressures representing 6 clinical conditions and calculated the mean arterial pressure using the formula (systolic + 2 × diastolic) d 3. These mean pressures were 57 mm Hg for shock, 70 mm Hg for low-normal blood pressure, 93 mm Hg for normal blood pressure, 107 mm Hg for high-normal blood pressure, and 133 mm Hg for severe hypertension. We used 14 mm Hg for the peripheral venous pressure.
From the American Red Cross we obtained and reconstituted 2 units of fresh-frozen plasma and 2 units of chilled red cells prepared with citrate phosphate dextrose anticoagulant. We constructed a model with an extracorporeal blood pump speed control (Bio Console 540, Medtronic Bio-Medicus, Eden Prairie, Minnesota) to circulate whole blood at a constant 4 L/min. We used a heat exchanger (Capiox Rx05 Baby RX, Terumo, Ann Arbor, Michigan) and a Haake heater maintained a constant 37°C by circulating heated water parallel to the whole-blood circuit. We measured pressure with a disposable pressure transducer (DTX Plus DT-4812, Becton Dickinson, Franklin Lakes, New Jersey) connected via a fluid-filled circuit; we verified its accuracy with a column of mercury, and we used a monitor (S/5, Datex-Ohmeda, Madison, Wisconsin) to calculate and display mean pressures.
We assigned a number 1–12 for the 6 pressures and 2 gauge combinations, and rolled dice to randomly select a pressure/needle combination for each puncture. We varied the partial clamping of a pair of hemostats to create the mean pressures to mimic the 6 clinical conditions, and we maintained the pressure within ± 3 mm Hg during sampling. We used 3-mL arterial blood samplers (Pro-Vent, Portex/Smith's Medical, St Paul, Minnesota), and preset them to 2 mL. We used the 22-guage/1.5-inch and 23-gauge/1-inch needles provided in the arterial blood sampling kit. We inserted the sampler and needle into the circuit at a 45° angle, via a 4.0-mm vascular access connector. We collected 10 samples at each pressure and both needle sizes. We used 2 stopwatches, and 2 individuals simultaneously timed the filling of the samplers. Timing began when blood was first visualized in the needle hub, and ended when the blood sealed the sampler and stopped filling. We accepted the filling times if they agreed within 10%, and we recorded the average of the 2 times.
We used statistics software (SPSS 17.0, SPSS, Chicago, Illinois) for the data analysis. To compare the filling times we calculated the mean ± SD, analysis of variance, with an alpha level of .05, and applied the Tukey honest significant difference post-hoc comparison. To identify statistically significant correlations between mean pressures and filling times for both needles combined, we calculated the Pearson correlation coefficient.
Results
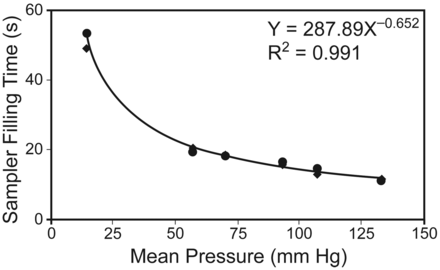
Table 1 presents the sampler filling times for each simulated mean pressure and needle combination. Table 2 shows the mean differences and probabilities of statistically significant differences in the sampler filling times between the 6 mean pressures. Figure 1 presents a scatter plot of the mean sampler filling times for the pressure/needle combinations. For all arterial pressures combined, the mean ± SD sampler filling time was 15.8 ± 0.4 s, and for all venous pressures combined the time was 51.4 ± 1.4 s (P < .001). With the 22-gauge/1.5-inch needles the mean ± SD sampler filling time was 22.2 ± 14.9 s, and with the 23-gauge/1-inch needles the time was 21.4 ± 13.1 s (difference not significant).
Sampler Filling Times for 6 Simulated Mean Pressures and 2 Gauge/Length Needle Combinations
Mean Differences and Probabilities of Statistically Significant Differences of Sampler Filling Times (in Seconds) Between 6 Simulated Mean Pressures
Sampler filling times versus pressures of 2 gauge-needle combinations. The dots represent the filling times with the 22-gauge/1.5-inch needles. The diamonds represent the filling times with the 23-gauge/1-inch needles.
The Pearson correlation coefficient of –0.86 (P = .01) indicates a strong negative correlation between mean blood pressure and sampler filling time. Thus, faster filling times occur at higher pressures. Table 2 displays the mean differences of the filling times and the probabilities of statistically significant differences. There was a statistically significant difference between simulated venous pressure and all simulated mean arterial blood pressure sampler filling times. There was a significant difference between filling times at 57 mm Hg for shock and normal pressures and higher. There was also a significant difference between filling times at 70 mm Hg for low-normal and both high-normal and severe hypertension, and between 93 mm Hg for normal and severe hypertension.
Discussion
Sampling for arterial blood gas analysis is drawing blood anaerobically from a peripheral artery via a single percutaneous needle puncture to obtain a blood specimen for direct measurement of partial pressures of carbon dioxide (PaCO2) and oxygen (PaO2), hydrogen ion activity (pH), total hemoglobin, oxyhemoglobin saturation, and the dyshemoglobins carboxyhemoglobin and methemoglobin.2 Although the pH and bicarbonate of venous blood have been shown to correlate well with the pH and bicarbonate of arterial blood, and may be used to identify patients with extreme hypercarbia, an arterial blood sample is necessary for assessing oxygenation and monitoring ventilation.7–9
Although clinical practice guidelines suggest that blood color and pulsatile blood flow can verify a successful arterial puncture, those are subjective indicators, whereas sampler filling time is an objective indicator and may be more reliable in clinical practice. Lower blood pressures increased sampler filling times, and the longest average filling time was 20.5 s with a mean arterial pressure of 57 mm Hg.
Based on the patient's mean arterial pressure, the therapist should have an informed expectation of the sampler filling time. Therefore, we suggest the therapist calculate the patient's mean arterial pressure prior to performing an arterial puncture. The scatter plot of the sampler filling times for the pressure/needle combinations may help to more accurately predict sampler filling times at other mean blood pressures, and may allow assessment of the effects of non-pressure-associated factors on sampling time in patients. With normal blood pressure the filling time was approximately 15 s. Informing the patient of the short expected filling time may reduce patient anxiety during the procedure. Filling time is probably linear, meaning that a 1-mL sample should be collected in less than 10 s, but this needs confirmation.
We expected needle resistance to affect filling time, but we found no differences. The needles we used in this study were the standard needles in the arterial blood sampling kit. Needle gauge refers to the external diameter of the needle; both needles were standard; neither was ultra-thin-walled. Since resistance to blood flow through a needle is directly related to the needle's length and inversely related to its gauge, and there were no differences in filling times between the needles, the difference in gauge was offset by the difference in length.
Limitations
Ours was a pilot study of the relationship between blood pressure and sampler filling time for a normal adult, using common arterial blood gas kit components. Additional variables that may affect this relationship, such as cardiac output, temperature, hematocrit, and arterial diameter, were intentionally controlled and not studied. Those variables, which also affect sampler filling time, provide the opportunity for future study. This was a laboratory experiment simulating an adult with a constant cardiac output, and differences between a laboratory model and real patients can be significant, especially during resuscitation and in various states of shock. Additionally, in a patient the needle's bevel may be partially occluded by the arterial wall or tissue, which would increase filling time. The results of this study need to be validated in a clinical trial. Additional limitations of our study include that the blood obtained from the American Red Cross came in separate components and had to be reconstituted. Hematocrit was not measured, so blood viscosity was not controlled. We also allowed minor variations in mean blood pressure and the 45o angle of entry.
Conclusions
Arterial sampler filling time is an objective indicator that may be used to confirm successful arterial puncture. The therapist should know the patient's mean arterial pressure before arterial puncture, which will enable the therapist to develop an informed expectation of the sampler filling time, which will help confirm successful arterial puncture. Confirming successful arterial puncture prior to blood analysis will expedite appropriate patient care decisions and reduce costs. Additional variables that also affect sampler filling time provide the opportunity for future research, and the results of this study need to be validated in a clinical trial.
Footnotes
- E-mail: kimberly.johnson2{at}osumc.edu.
-
The authors have disclosed no conflicts of interest.
-
Kimberly L Johnson RRT presented a version of this paper at Current Concepts in Respiratory Care, held in Columbus, Ohio, April 3, 2009, at the Denman Undergraduate Research Forum, held in Columbus, Ohio, May 13, 2009, and at the 55th International Respiratory Congress of the American Association for Respiratory Care, held December 5–8, 2009, in San Antonio, Texas.
- Copyright © 2011 by Daedalus Enterprises Inc.