Abstract
Noninvasive ventilation (NIV) for acute respiratory failure has gained much academic and clinical interest. Despite this, NIV is underutilized. The evidence strongly supports its use in patients presenting with an exacerbation of COPD and in patients with acute cardiogenic pulmonary edema. As reviewed in this paper, there is now evidence supporting or not supporting the use of NIV in various other presentations of acute respiratory failure. It is important not only to know when to initiate NIV, but also when this therapy is failing. Whether NIV in the setting of acute respiratory failure can be managed appropriately outside the ICU setting is controversial. Although a variety of interfaces are available, the oronasal mask is the best initial interface in terms of leak prevention and patient comfort. Some critical care ventilators have NIV modes that compensate well for leaks, but as a group the ventilators that are designed specifically for NIV have better leak compensation. NIV should be part of the armamentarium of all clinicians caring from patients with acute respiratory failure.
Introduction
Over the past 20 years there has been increasing interest in the use of noninvasive ventilation (NIV). During that time, there have been scores of published randomized controlled trials (RCTs), observational studies, and case reports. The 173 references cited in this paper represent a small fraction of what has been published on this topic. These papers have dealt with patient selection, interfaces, the ventilator and ventilator settings, and implementation protocols. More recently there have been several informative narrative reviews1,2 and systematic reviews3,4 published on the use of NIV in acute care. For many respiratory therapists and physicians, the growth in use of NIV has occurred within the years of our careers. Indeed, in 1977 the former editor in chief of Respiratory Care, Philip Kittredge, wrote, “CPAP is no longer a new therapy, nor, alas, is the strapped positive-pressure breathing mask a new device. It is, rather, as antiquated as it is inhumane and unsafe … A patient who is sick enough to need CPAP is sick enough to need an endotracheal tube.”5 This was the prevalent opinion of many of us practicing at that time. In this review, I will address contemporary issues related to patient selection, equipment selection, and implementation strategies for NIV in the acute care setting.
CPAP Versus Noninvasive Ventilation
The terms CPAP and noninvasive ventilation (NIV) are sometimes used interchangeably. They are, however, distinctly different. With noninvasive CPAP, a face mask or other interface is used to apply a pressure greater than atmospheric to the proximal airway. The result is splinting open the upper airway, an increase in lung volume, and an increase in intrathoracic pressure. With CPAP there is no inspiratory muscle unloading; in fact, tidal ventilation is completely dependent on the respiratory muscles with CPAP. NIV, on the other hand, applies a pressure during the inspiratory phase greater than the pressure applied during exhalation. Thus, NIV unloads the respiratory muscles and can provide complete respiratory support.
Patient Selection
COPD Exacerbation
The most robust evidence supporting the use of NIV is arguably for the patient with exacerbation of COPD. A Cochrane review6 included 14 RCTs comparing NIV plus usual care versus usual care alone. The use of NIV decreased the need for intubation, with a relative risk (RR) of 0.41 (95% CI 0.33–0.53); this translates into a number needed to treat (NNT) of 4 (95% CI 4–5). More important, NIV decreased mortality, with a RR of 0.52 (95% CI 0.35–0.76), which is a NNT of 10 (95% CI 7–20).
Chandra et al7 used data from the Healthcare Cost and Utilization Project's Nationwide Inpatient Sample to assess the pattern and outcomes of NIV use for COPD exacerbations from 1998 to 2008. The use of NIV increased significantly over time among patients hospitalized for COPD exacerbations, while the need for intubation and in-hospital mortality declined. Of concern, however, was the rising mortality rate in a small but expanding group of patients requiring invasive mechanical ventilation after NIV. The authors propose 2 explanations for the high mortality rate in patients requiring invasive mechanical ventilation after initiation of NIV: increasing the use of NIV in patients who are difficult to ventilate, and continuation of NIV despite a lack of early improvement. The design of this study, however, does not provide evidence to confirm or refute either of these explanations.
Clinical practice guidelines published by the Canadian Critical Care Trials Group recommend NIV in addition to usual care in patients who have a severe exacerbation of COPD (pH < 7.35 and relative hypercapnia), with an 1A level of evidence.3 Moreover, they state that NIV should be the first option for ventilatory support for patients with a severe exacerbation of COPD. The role of NIV in patients with milder exacerbations of COPD is unclear, with one study reporting poor tolerance in such subjects.8
Cardiogenic Pulmonary Edema
There is also robust evidence supporting the use of NIV for acute cardiogenic pulmonary edema. In a Cochrane review9 including 21 studies and 1,071 subjects, it was reported that NIV, compared to standard medical care, significantly reduced the need for endotracheal intubation, with a RR of 0.53 (95% CI 0.34–0.83) and a NNT of 8. There was also a significant reduction for hospital mortality, with a RR of 0.6 (95% CI 0.45–0.84) and NNT of 13. Compared to standard medical care, there was no significant increase in the incidence of acute myocardial infarction with NIV (RR 1.24, 95% CI 0.79–1.95), as had been a concern following an earlier RCT.10 In a meta-analysis by Winck et al,11 7 studies of NIV compared to CPAP in subjects with acute cardiogenic pulmonary edema showed a nonsignificant difference between the 2 therapies. In a subgroup analysis in the same meta-analysis, NIV did not lead to better outcomes than CPAP in studies including more subjects with hypercapnia. It has long been known that CPAP can result in important physiologic improvements in this patient population, such as a reduction in breathing frequency and PaCO2, and an improvement in PaO2/FIO2.12 The Canadian clinical practice guidelines recommend NIV as the first option for ventilatory support for patients with cardiogenic pulmonary edema, and suggest that CPAP is just as effective as NIV in this patient population.3 Other systematic reviews and narrative reviews reached similar conclusions.13–19
Post-Extubation
NIV can be used in the post-extubation period to shorten the duration of invasive ventilation, to prevent extubation failure, and to rescue a failed extubation.20–22 Burns et al23,24 conducted a systematic review and meta-analysis of randomized and quasi-randomized controlled trials to evaluate the evidence for extubation with immediate application of NIV, compared with continued invasive weaning. Compared with invasive weaning, NIV was associated with reduced mortality, lower rates of ventilator-associated pneumonia (VAP), fewer ICU and hospital days, shorter total duration of ventilation, and shorter duration of invasive ventilation. The authors concluded that use of NIV to allow earlier extubation should be used in patients with COPD in a highly monitored environment. The results of a small study by Vaschetto et al25 suggest that NIV may also be useful to facilitate discontinuation of mechanical ventilation in selected patients with resolving hypoxemic respiratory failure. In subjects with neuromuscular disease, Bach et al26 reported successful extubation in many of those who did not meet criteria for ventilator discontinuation. Although this was not an RCT, it illustrates that, in patients with acute respiratory failure secondary to neuromuscular disease who require intubation, extubation can occur directly to NIV rather than performing a tracheostomy.
Early application of NIV, immediately after extubation, can be effective in preventing post-extubation respiratory failure in those at risk. Results of a meta-analysis showed that NIV decreases reintubation rate and ICU mortality in subjects who are at risk for developing post-extubation respiratory failure.27 The studies by Nava et al28 and Ferrer et al29 inform the selection of patients at risk for extubation failure and likely to benefit from the use of NIV in this setting (Table 1). However, routine use of NIV immediately after extubation is not recommended. Su et al30 conducted a multicenter RCT in 406 subjects who tolerated an SBT and were subsequently extubated. Subjects were randomized to NIV or standard medical therapy. There were no differences in extubation failure or ICU or hospital mortality. Thus, preventive use of NIV after extubation routinely in all patients who pass an SBT is not beneficial in decreasing extubation failure rate or the mortality rate.
In subjects with established post-extubation respiratory failure, 2 RCTs have evaluated the role of NIV.31,32 In the study by Keenan et al32 comparing NIV to standard medical therapy to rescue extubation failure, there was no difference in the rate of reintubation, hospital mortality, or duration of mechanical ventilation or ICU or hospital stay. Esteban et al31 conducted a multicenter RCT to evaluate the effect of NIV on mortality for subjects who developed respiratory failure after extubation. There was no difference between the NIV group and the standard-therapy group in the need for reintubation. Of concern was the higher ICU mortality rate in the NIV group, compared with the standard-therapy group. The available evidence suggests that, in patients who do not have COPD, NIV is not effective in treating established post-extubation respiratory failure.
The study by Girault et al33 helps to inform the use of NIV in the post-extubation period. They evaluated NIV effectiveness as an early extubation technique in difficult-to-wean patients. This was a multicenter RCT conducted in 13 ICUs enrolling subjects with chronic respiratory failure and hypercapnia (most with COPD) who were intubated for acute respiratory failure and who failed their first SBT. Subjects were assigned to 3 groups: conventional invasive weaning group, extubation followed by standard oxygen therapy, or NIV. NIV was permitted as rescue therapy for both non-NIV groups if post-extubation respiratory failure occurred. The reintubation rates were 30%, 37%, and 32% for the invasive weaning, oxygen-therapy, and NIV groups, respectively. The weaning failure rates, including post-extubation respiratory failure, were 54%, 71%, and 33%, respectively. The success rates for rescue NIV in the invasive and oxygen-therapy groups were 45% and 58%, respectively. Other than a longer weaning time in the NIV group than in the invasive group, no significant outcome difference was observed between the groups. Although there was no significant difference in the reintubation rates between the 3 weaning strategies, this study demonstrated that NIV improves weaning results by reducing the risk of post-extubation acute respiratory failure. It is important to note that these results also suggest that rescue NIV might be useful to avoid reintubation when post-extubation respiratory failure occurs.
Immunocompromised Patients
Immunocompromised patients who develop acute respiratory failure often require respiratory support. In such patients, endotracheal intubation is associated with substantial mortality.34 The benefit of NIV in immunocompromised patients with acute respiratory failure has been evaluated in 2 RCTs and a number of observational studies. Antonelli et al35 evaluated 40 subjects following solid-organ transplantation who developed hypoxemic respiratory failure and were randomized to receive NIV or oxygen therapy. Subjects treated with NIV had better oxygenation and lower rates of intubation and mortality. Hilbert et al36 randomized 52 hypoxemic immunosuppressed subjects with acute respiratory failure and pneumonia to NIV or supportive oxygen only, and reported a reduction in the need for endotracheal intubation and hospital mortality for the group receiving NIV. Squadrone and colleagues37 reported that early use of CPAP on a hematological ward in subjects with early changes in respiratory parameters prevents evolution to acute lung injury requiring mechanical ventilation and ICU admission. Currently available evidence supports NIV as the first-line approach for managing mild to moderately severe respiratory failure in selected patients with immunosuppression.34 In this patient population, factors found to be associated with NIV failure were breathing frequency while receiving NIV, longer delay between admission and the first use of NIV, need for vasopressors or renal replacement therapy, and the presence of ARDS.38
ARDS
The use of NIV in patients with ARDS is controversial. Most studies that have addressed this patient population enrolled subjects who did not have indications for immediate endotracheal intubation. Zhan et al39 assessed the safety and efficacy of NIV in 40 subjects with mild ARDS. Subjects were randomly allocated to receive either NIV or oxygen. NIV was associated with a lower breathing frequency and improved PaO2/FIO2 over time, and the proportion of patients requiring intubation was significantly lower in the subjects receiving NIV. Ferrer et al40 randomized 105 subjects with severe hypoxemic respiratory failure to receive either NIV or high FIO2. The respiratory-failure etiologies were mostly pneumonia and cardiogenic pulmonary edema, but there were 15 subjects with ARDS. NIV prevented intubation, reduced the incidence of septic shock, and improved survival. In a prospective observational study, Agarwal et al41 evaluated the role of NIV for hypoxic respiratory failure. Subjects were classified into 2 groups: ARDS and other causes. They reported that 12 of the 21 ARDS subjects needed intubation, versus 7 of the 19 in the other group. By univariate logistic regression, the only factor associated with NIV failure was the baseline PaO2/FIO2.
In a prospective multicenter cohort study, Antonelli et al42 investigated factors related to NIV failure; the highest failure rate was observed in the subjects with ARDS. In another observational study, only 17% of the subjects admitted with ARDS were successfully treated with NIV.43 In the Canadian experience with H1N1 ARDS, 33% of subjects initially received NIV, but the failure rate for NIV was 85%.44 Agarwal et al45 conducted a meta-analysis in which they included 13 studies with a total of 540 subjects. The pooled intubation rate was 48% and the pooled mortality rate was 35%. However, few of the studies analyzed were randomized, and the subjects had heterogeneous underlying pathologies (eg, community-acquired pneumonia, sepsis, and near-drowning), which makes it difficult to draw conclusions related to ARDS. The available evidence suggests caution in the use NIV in ARDS.
NIV should be used very cautiously, and perhaps not at all, in patients with ARDS who have shock, metabolic acidosis, or profound hypoxemia. Rana et al46 assessed the outcome of subjects with ARDS initially treated with NIV. All those with shock failed NIV. Metabolic acidosis (odds ratio 1.27, 95% CI 1.03–0.07 per unit of base deficit) and severe hypoxemia (odds ratio 1.03, 95% CI 1.01–1.05 per unit decrease PaO2/FIO2) predicted NIV failure. In patients who failed NIV, the observed mortality was higher than the Acute Physiology and Chronic Health Evaluation (APACHE) predicted mortality (68% vs 39%, P < .01).
Acute Asthma
In 1996, Meduri et al47 published an observational study of the use of NIV in 17 episodes of acute asthma. The authors of this report were enthusiastic about the use of NIV in this population, concluding that NIV appears highly effective in correcting gas exchange abnormalities in the setting of acute asthma. However, in the absence of RCTs, many clinicians were skeptical of the use of NIV in this setting. In fact, the authors of a Cochrane review published in 2005 concluded that the application of NIV in subjects suffering from status asthmaticus, despite some interesting and very promising preliminary results, remains controversial. However, several more recent RCTs might better inform the use of NIV for severe acute asthma.
Soroksky et al48 randomized 30 subjects with severe acute asthma to conventional therapy or NIV. NIV significantly improved lung function; 80% of the subjects in the NIV group reached the predetermined primary end points of a 50% increase in FEV1 compared to baseline, versus 20% of control subjects. Hospitalization was required for 18% in the NIV group, as compared with 63% in the control group. The authors concluded that, in patients with severe acute asthma, the addition of NIV to conventional treatment improves lung function, alleviates the exacerbation faster, and significantly reduces the need for hospitalization.
Gupta and colleagues49 randomized 53 subjects with severe acute asthma to NIV or standard medical therapy. There was a significant improvement in breathing frequency, FEV1, and PaO2/FIO2, but not pH or PaCO2, in both the groups, and no significant difference between the 2 groups. The mean dose of inhaled bronchodilator was significantly less in the NIV group. There were 4 instances of standard-medical-therapy failure, but none in the NIV group. There was no mortality in either of the groups. The authors concluded that, in patients with severe acute asthma, the addition of NIV to standard medical therapy accelerates the improvement in lung function, decreases the inhaled bronchodilator requirement, and shortens the ICU and hospital stay. Murase et al50 conducted a retrospective cohort study of the use of NIV for acute asthma. There were 50 subjects from the pre-NIV period and 57 events from the post-NIV period. In the pre-NIV period, 9 cases were treated primarily by endotracheal intubation. In the post-NIV period, 17 cases were treated primarily by NIV, with intubation used in only 2 subjects. No deaths occurred as a consequence of asthma exacerbation.
Basnet et al51 evaluated the safety, tolerability, and efficacy of early initiation of NIV in addition to standard of care in the management of 20 children (1–18 y of age) admitted to a pediatric ICU with status asthmaticus. Improvement in clinical asthma score was significantly greater in the NIV group, compared to the standard therapy group, at 2 h, 4–8 h, 12–16 h, and 24 h after initiation of therapy. A significant decrease in breathing frequency at ≥ 24 h and oxygen requirement after 2 h was noted in the NIV group. Fewer children in the NIV group required adjunct therapy, compared to the standard group (11% vs 50%). There were no major adverse events related to NIV. In terms of tolerance, 9 of 10 subjects tolerated NIV. The authors concluded that early initiation of NIV, in conjunction with short acting β agonists and systemic steroids, is safe, well tolerated, and effective in the management of children with status asthmaticus.
In a narrative review, Soroksky et al52 point out that reports of NIV use in patients with severe acute asthma are scarce, and its use in this setting remains controversial. The available studies involve small numbers of patients. In an editorial, Scala53 suggests that NIV might be applied with different aims in the time-course of an episode of severe acute asthma (Fig. 1):
As an alternative to intubation in patients who have failed a trial of standard medical treatment
To prevent intubation in patients with mild-to-moderate acute respiratory failure who do not need immediate ventilatory support
To prevent acute respiratory failure in patients who do not have substantial impairment of gas exchange
To accelerate bronchodilation in patients who do not need mechanical ventilation
Potential goals of noninvasive ventilation (NIV) in severe acute asthma. ARF = acute respiratory failure. (From Reference 53.)
Each of these points is hypothesis generating, and sufficient evidence to make recommendations is lacking. Because the mortality rate for asthma should be very low without the use of NIV, further reduction in mortality may not be an appropriate end point of NIV in patients with acute asthma, unlike studies of COPD exacerbation and acute cardiogenic pulmonary edema.
Community-Acquired Pneumonia
The benefit of NIV in patients with pneumonia is controversial due to high failure rates.40,54–56 Carrillo et al57 assessed the characteristics and predictors of outcome of subjects with community-acquired pneumonia and severe acute respiratory failure treated with NIV. NIV failed more frequently in subjects with de novo acute respiratory failure (46%) than subjects with previous cardiac or respiratory disease (26%). Worsening radiographic infiltrate 24 h after admission, maximum Sepsis-Related (or Sequential) Organ Failure Assessment (SOFA) score and, after 1 h of NIV, higher heart rate and lower PaO2/FIO2 and bicarbonate independently predicted NIV failure. SOFA, NIV failure, and older age independently predicted hospital mortality. Longer duration of NIV before intubation was associated with decreased hospital survival in subjects with de novo acute respiratory failure, but this was not observed in subjects with previous cardiac or respiratory disease. The authors concluded that successful NIV was strongly associated with better survival. But if predictors for NIV failure are present, avoiding delayed intubation of patients with de novo acute respiratory failure may reduce mortality.
Do Not Intubate or Do Not Resuscitate
Few data are available on NIV in patients who have elected specific limits on life support and treatments, such as patients with do-not-intubate (DNI) orders, and patients who are near the end of life and will receive comfort measures only (CMO). Sinuff et al58 reported that, for subjects with DNI orders, many physicians use NIV, and many respiratory therapists are asked to initiate NIV, most often to treat COPD and cardiogenic pulmonary edema.
Levy et al59 evaluated the outcomes of 114 subjects who had a DNI status and received NIV. Of these, 43% survived to discharge. Subjects with congestive heart failure had a significantly better survival rate than those with COPD, cancer, pneumonia, or other diagnoses. A stronger cough and being conscious were also associated with a higher probability of survival. In 137 episodes of acute respiratory failure, Schettino et al60 reported that NIV successfully reversed acute respiratory failure and prevented hospital mortality in subjects who were DNI with COPD and cardiogenic pulmonary edema. However, NIV was less beneficial in subjects with post-extubation failure, hypoxemic respiratory failure, or end-stage cancer. The results of these studies suggest that some patients who are DNI, particularly those with diagnoses such as congestive heart failure or COPD, who have a strong cough, and are awake may have a good prognosis with NIV. Patients for whom intubation in the late stages of chronic illness is inappropriate should be offered a trial of NIV, as this may allow them to survive an otherwise fatal episode of respiratory failure.
The Society of Critical Care Medicine charged a task force with developing an approach for considering use of NIV for patients who are DNI.61 They suggested that the use of NIV for patients with acute respiratory failure can be classified into 3 categories: NIV as life support with no preset limitations on life-sustaining treatments; NIV as life support when patients and families have decided to forego endotracheal intubation; and NIV as a palliative measure when patients and families have chosen to forego all life support, receiving CMO. The task force suggests that NIV should be applied after careful discussion of the goals of care, with explicit parameters for success and failure, by experienced personnel, and in appropriate healthcare settings. Kacmarek62 suggests that the most critical issue regarding NIV in DNI and CMO patients is informed consent. The patient must be informed of the risks and potential benefits of NIV, and must consent to NIV. If informed consent and control of care decisions are assured, then NIV may be appropriate in DNI and CMO patients, to reverse an acute respiratory failure that is not necessarily life-terminating, or to improve patient comfort, or to delay death.
There are a number of unanswered questions related to the use NIV in patients who are DNI or CMO.63 It is not known whether palliative NIV increases duration of life or if it extends the dying process. Qualitative observational data are needed to identify the benefits of palliative NIV, such as improvement of family experience, patient's well being, quality of end-of-life care, family satisfaction, and the global clinician's perspective. It is also unclear whether palliative NIV should be performed in incapacitated patients in order either to improve survival or to alleviate symptoms of respiratory distress.
Pre-oxygenation Before Intubation
Baillard et al64 evaluated whether NIV is more effective at reducing desaturation than usual pre-oxygenation during orotracheal intubation in hypoxemic subjects. Pre-oxygenation was performed before a rapid sequence intubation, for a 3 min period, using a bag-valve mask (control group) or pressure support ventilation (PSV) delivered by an ICU ventilator through a face mask (NIV group). At the end of pre-oxygenation, SpO2 was higher in the NIV group, as compared with the control group (98% vs 94%); 46% of subjects in the control group and 7% in the NIV group had an SpO2 below 80%. Five minutes after intubation, SpO2 values were still better in the NIV group, as compared with the control group. In 66 morbidly obese subjects, Futier et al65 used either 5 min of conventional pre-oxygenation with spontaneous breathing of 100% oxygen, NIV, or NIV followed by a recruitment maneuver. At the end of pre-oxygenation, PaO2 was higher in the NIV and NIV with recruitment maneuver groups. After the onset of invasive mechanical ventilation, PaO2 and lung volume were greater in the NIV groups. Thus it appears that NIV improves oxygenation and lung volume in morbidly obese patients, compared with conventional pre-oxygenation.
Post-Operative Respiratory Failure
Several recent reviews have addressed the use of NIV in post-operative care.66–69 Jaber et al69 suggest that there are 2 potential goals of NIV in the post-operative period: 1) to prevent acute respiratory failure (prophylactic treatment) or, 2) to treat acute respiratory failure and avoid reintubation (curative treatment). Chiumello et al66 conducted a systematic review of 29 studies including 2,279 subjects. There were 9 studies that evaluated NIV in post-abdominal surgery, 3 in thoracic surgery, 8 in cardiac surgery, 3 in thoraco-abdominal surgery, 4 in bariatric surgery and 2 in post solid organ transplantation. The use of NIV improved arterial blood gases in 15 of the 22 studies evaluating prophylactic uses and in 4 of the 7 studies evaluating curative uses. NIV reduced the intubation rate in 11 of the 29 studies, but improved survival in only 1 study. These authors concluded that, despite limited data and the necessity of additional RCTs, NIV should be considered as a prophylactic and curative tool to improve gas exchange in post-operative patients.
Auriant et al70 randomized 24 subjects with acute hypoxemic respiratory insufficiency after lung resection surgery to NIV or standard therapy. Despite the small sample size, there was a significant difference in the need for endotracheal intubation in the subjects who received NIV (50% in the group who did not receive NIV vs 21% in the NIV group). Perrin et al71 evaluated the use of NIV administered prophylactically pre- and post-operatively. Subjects followed standard treatment without or with NIV for 7 days at home before surgery and during 3 days post-operatively. Oxygenation was significantly better in the NIV group for the first 3 post-operative days, and hospital stay was significantly shorter for the NIV group. In an observational prospective survey, Lefebvre et al72 evaluated the feasibility and efficacy of early NIV in subjects with acute respiratory failure following lung resection surgery. The overall success rate of NIV was 85%. Riviere and colleagues73 reported the following variables associated with NIV failure following lung surgery: tachypnea, higher Sequential Organ Failure Assessment score, number of bronchoscopies performed, and number of hours spent on NIV. A concern with the use of NIV following thoracic surgery is the risk of air leak with positive-pressure ventilation, but this has not been reported in the studies to date.
CPAP may be effective in patients with post-operative atelectasis. In an RCT of 209 subjects who developed acute hypoxemia after elective major abdominal surgery, Squadrone and colleagues74 assigned subjects to receive oxygen or CPAP. Subjects who received CPAP had a lower intubation rate, lower pneumonia rate, and spent fewer days in the ICU than subjects treated with oxygen alone. Zarbock et al75 randomized 500 subjects following extubation to standard treatment or prophylactic CPAP for at least 6 h. Hypoxemia, pneumonia, and reintubation rate were reduced in subjects receiving prophylactic CPAP. The readmission rate to the ICU was also reduced in subjects receiving prophylactic CPAP.
Sleep-disordered breathing is common in post-operative patients.76–81 Practice guidelines for the peri-operative management of patients with obstructive sleep apnea are available and should be considered by clinicians caring for these patients.78 For patients using CPAP for obstructive sleep apnea, it is important that this therapy is available in the immediate post-operative period.
Obesity Hypoventilation Syndrome
The prevalence of extreme obesity has markedly increased. Obesity hypoventilation syndrome (OHS) is the triad of obesity, daytime hypoventilation, and sleep-disordered breathing. An important treatment of OHS includes the use or either CPAP or NIV in ambulatory patients as well as those with acute respiratory failure.82,83 Priou et al84 found that long-term NIV was an effective and well tolerated treatment of OHS when initiated in the acute care setting. When patients with OHS are intubated for acute respiratory failure, it is important to resume CPAP or NIV following extubation.
Carrillo et al85 prospectively assessed 173 subjects with OHS and 543 subjects with COPD, all with acute hypercapnic respiratory failure. Patients with OHS were older, were more frequently female, had fewer late NIV failures, had lower hospital mortality, and had higher 1-year survival (odds ratio 1.83, 95% CI 1.24–2.69, P = .002). However, survival rates adjusted for confounders, NIV failure, stay, and hospital re-admission, were each similar between the groups. Among patients with COPD, obesity was associated with less late NIV failure and hospital readmission. The authors concluded that patients with OHS and acute hypercapnic respiratory failure treated with NIV have similar efficacy and better outcomes than patients with COPD. NIV appears at least as effective in acute OHS as in COPD. Treatment of OHS requires a multimodal therapeutic approach, including NIV at home as well as during acute care; rehabilitation programs with physical training, weight loss, and lifestyle changes; and appropriate medication to further control cardiovascular risk factors.86
Bronchoscopy
Flexible bronchoscopy is often necessary in severely ill hypoxemic patients with comorbidities that increase the risk of bronchoscopy-related complications. NIV might decrease the risk of these complications in patients with severe refractory hypoxemia, post-operative respiratory distress, or severe emphysema. NIV might also prevent hypoventilation in patients with obstructive sleep apnea and OHS who require bronchoscopy, and may assist in the bronchoscopic evaluation of patients with expiratory central-airway collapse.87 NIV-assisted bronchoscopic lung biopsy may be useful to obtain a diagnosis in hypoxemic subjects with diffuse lung infiltrates (Fig. 2).88 Despite the number of reports describing the use of NIV during bronchoscopy,87–96 this approach should be reserved for centers with extensive experience in NIV.
Bronchoscope inserted through the swivel adaptor of a face mask for noninvasive ventilation. (From Reference 88.)
When to Start, When to Stop, When to Transfer, When to Sedate, When to Wean
When to Start
Identification of patients likely to benefit from NIV can be considered a 2-step process. In the first step the patient should be determined to need mechanical ventilation, as identified by signs of respiratory distress, tachypnea, accessory muscle use, and acute respiratory acidosis. These patients should ideally have a diagnosis where the evidence has shown benefit for use of NIV (eg, COPD, acute cardiogenic pulmonary edema). In the second step, the patient should have no exclusions for NIV, such as the need for an artificial airway for airway protection, inability to fit an interface, high severity of illness (eg, respiratory arrest), an uncooperative patient who will not allow placement of the interface, and a diagnosis where it has been shown that NIV is not effective (eg, severe ARDS). Patient wishes should also be considered; some patients may elect not to receive NIV.
When to Stop
Recognition that NIV is failing is an important, but often overlooked, part of the management of NIV. The reported NIV failure rate is 5–40%.97 Some patients fail due to progression of the disease process. Greater clinician experience and expertise with the application of NIV are associated with a higher success rate.98 Some patients do not obtain adequate ventilation with NIV and therefore require intubation. It is not always apparent which patients will initially benefit from NIV, but recognized risk factors for NIV failure are shown in Table 2.99 Confalonieri et al100 found that subjects likely to fail NIV had more severe respiratory acidosis, a lower level of consciousness, were older, were more hypoxemic, and had a higher breathing frequency on presentation. Clinical signs that are only equivocal on presentation become more definitively predictive of failure if they persist after 2 h of NIV. Thus, it is important to assess clinical trajectory after 1–2 h of initiation of NIV to identify response. However, even on presentation, subjects who have a pH < 7.25, an APACHE II score > 29, and a Glasgow coma score < 11 have failure rates ranging from 64% to 82%. Berg et al101 evaluated the ability of the rapid shallow breathing index (RSBI), the ratio of breathing frequency (breaths/min) to tidal volume (L), to predict NIV failure. Of 83 subjects with an RSBI ≤ 105, 31% required intubation, compared to 55% with an RSBI > 105 (multivariate odds ratio 3.70, 95% CI 1.14–11.99). One reason for NIV failure is selection of inappropriate ventilator settings, and it is unknown whether the subjects in this study with an elevated RSBI could also have benefitted from an increase in NIV settings.
Risk Factors for Noninvasive Ventilation Failure
When to Transfer to the ICU
The optimal location to apply NIV is a matter of debate.99 Although some have argued that all acute care NIV should be initiated in the ICU, this is often impractical because ICU beds are unavailable. The ability to safely administer NIV differs among various sites, even in the same hospital. Choosing the appropriate site for NIV requires consideration of the patient's need for monitoring, the monitoring capabilities of the unit, the technical and personnel resources available (nursing and respiratory therapy), and the skill and experience of the staff. In many hospitals, NIV is initiated in the emergency department, after which the patient is transferred to the ICU. Step-down units can be good locations for NIV. With ICU beds at a premium, many hospitals are forced to manage some patients receiving NIV on general wards. This can be done with more stable patients with suitable monitoring if the staff is adequately trained in the technique and available throughout the 24-h period. The ideal location for NIV varies from country to country and from hospital to hospital, dictated by local factors.102
In an observational study, Farha et al103 evaluated the use of NIV on general nursing units at the Cleveland Clinic, and reported that NIV was frequently used on the regular hospital ward and that the success rate was similar to that reported when NIV is used in the ICU. Kacmarek and Villar104 suggest that it is possible to manage many patients requiring NIV for acute respiratory failure outside the ICU. But they also urge caution: patients need to be carefully selected, and appropriate preparations need to be made in the units caring for these patients, to ensure their safety. They further state that it is not acceptable to assume that any patient care unit is capable of caring for patients in acute respiratory failure who require NIV. At the Massachusetts General Hospital we have adopted a checklist (Fig. 3) to identify patients who should be transferred to the ICU after NIV is initiated in the general care units. This checklist is completed by clinicians (physicians, respiratory therapists, and nurses) who meet (huddle) shortly after the initiation of NIV and then again after 2 hours. Although we have not formally evaluated this program, anecdotally it has been found useful to inform the decision regarding transfer of patients to the ICU. For safety, it is also important that the patient is transferred while receiving NIV, and many ventilators for NIV have internal batteries to facilitate this.
Huddle form and checklist, as used at the Massachusetts General Hospital.
When to Sedate
Some patients are intolerant of NIV, becoming anxious when the interface is applied. However, clinicians are usually reluctant to administer sedative agents, fearing that these might decrease respiratory drive and consciousness, which could lead to NIV failure. Devlin et al105 conducted a survey to characterize current practices and attitudes regarding sedation during NIV. Of physicians who responded, 15%, 6%, and 28% never used sedation, analgesia, or hand restraints, respectively, at any time for patients receiving NIV. Sedation, analgesia, and hand restraints were more commonly used in North America than in Europe. A benzodiazepine alone was the most preferred (33%), followed by an opioid alone (29%).
Remifentanil is a potent short-acting synthetic opioid used for pain relief and sedation (analgosedation). Constantin et al106 assessed the feasibility and safety of remifentanil-based sedation in 13 subjects with NIV failure due to discomfort and/or refusal to continue the therapy. Subjects were sedated to a Ramsay scale of 2–3 by a continuous infusion of remifentanil during NIV. Subject tolerance improved, PaO2/FIO2 increased, breathing frequency decreased, and PaCO2 decreased with remifentanil-based sedation. The authors concluded that that remifentanil-based sedation is safe and effective in the treatment of NIV failure due to low tolerance. Rocco et al107 reported the use of remifentanil-based sedation in 36 subjects intolerant of NIV, and concluded that this sedation protocol can decrease the rate of failure in subjects with intolerance to NIV.
Dexmedetomidine has favorable respiratory and cardiovascular pharmacologic properties at therapeutic doses, and thus it may be an ideal pharmacologic agent for sedation of patients intolerant of NIV. Akada et al108 conducted a prospective clinical investigation of the effect of dexmedetomidine in 10 subjects in whom NIV was difficult because of agitation. All subjects were successfully weaned from NIV, and the respiratory state was not worsened. The authors concluded that dexmedetomidine is an effective sedative drug for patients with NIV. Several case reports have also reported successful use of dexmedetomidine in patients intolerant of NIV.109,110
When to Wean
There is usually no formal approach to weaning patients from NIV. Typically, the interface will be removed per patient request, to provide facial hygiene, or to administer oral medications. If the patient deteriorates when NIV is interrupted, the therapy is resumed, but otherwise NIV is discontinued. Duan et al111 conducted an RCT in which respiratory therapists screened subjects daily for readiness to discontinue NIV (64% with COPD) and, if appropriate per the screen, initiated weaning according to a protocol. In the physician-directed weaning group the weaning attempt was initiated according to physicians' orders. Compared with physician-directed weaning, therapist-protocol-directed weaning reduced the duration of NIV and the duration of the ICU stay.
Technical Aspects
Which Interface?
The interface distinguishes NIV from invasive ventilation112–114 (Table 3). A variety of interfaces are commercially available for NIV (Table 4 and Fig. 4).115 Fraticelli et al116 evaluated 4 interfaces for NIV. Despite differences in internal volume, no apparent dead space effect was observed on minute ventilation, work of breathing, or PaCO2. NIV was uniformly successful in reducing indexes of respiratory effort, regardless of the interface. Leaks and asynchrony were greater with the mouthpiece device, and comfort with this interface was deemed poor for most patients. The authors concluded that, with the exception of the mouthpiece, interfaces may be interchangeable in clinical practice, provided adjustment of the ventilatory device parameters is performed. Girault et al117 assessed the influence of initial mask choice on the effectiveness and tolerance of NIV in subjects with hypercapnic acute respiratory failure. Mask failure occurred significantly more often in the nasal mask group due to major leaks. The authors concluded that the oronasal mask should be the first-line strategy in the initial management of hypercapnic acute respiratory failure with NIV. Kwok et al118 randomly assigned subjects needing NIV for acute respiratory failure to either a nasal or an oronasal mask. Although both masks performed similarly with regard to improving gas exchange and avoiding intubation, the nasal mask was less well tolerated than the oronasal mask. Anton et al119 assessed the efficacy and subject tolerance of nasal and oronasal masks in subjects with COPD exacerbation. The group that used the oronasal mask had a greater decrease in breathing frequency, with no other differences between the interfaces. The authors concluded that NIV improves blood gases and respiratory effort indices regardless of the type of mask used.
Advantages and Disadvantages of Various Types of Interfaces for Noninvasive Ventilation
Desirable Characteristics of an Interface for Noninvasive Ventilation
Interfaces for noninvasive ventilation. Top (left to right): nasal mask, nasal pillows, oronasal mask, hybrid mask. Bottom (left to right): oral mask, total face mask, helmet. (From Reference 115.)
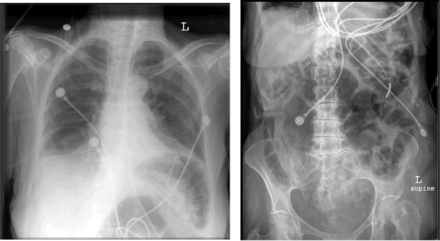
Leaks through the mouth are common with a nasal interface. This can affect comfort, result in dry mouth and in less effective ventilation,120,121 affect patient-ventilator interaction (trigger and cycle), and disrupt sleep architecture.122 A chin strap can be tried,121,123 but use of an oronasal mask may be more effective. A concern with the use of an oronasal mask is aspiration should regurgitation occur, but this is rare. Asphyxiation in the event of a ventilator malfunction is also a concern, but commercially available oronasal masks are often equipped with anti-asphyxia valves. Aerophagia commonly occurs with NIV, but this is usually benign, because the airway pressures are less than the esophageal opening pressure. Gastric insufflation can be severe (Fig. 5), but this is usually the result of inspiratory pressure settings that are too high. A gastric tube is not routinely necessary for mask ventilation.
Chest and abdomen radiographs of a patient who developed severe gastric insufflation while receiving noninvasive ventilation.
A potential problem with nasal and oronasal masks is facial skin breakdown, which most commonly occurs on the bridge of the nose. Some commercially available face masks can produce substantial pressure on the bridge of the nose.124 Nasal skin breakdown has been estimated to occur in 5–20% of applications of NIV.115 This is of particular concern because stage 3 or 4 pressure ulcers acquired after hospital admission are considered serious reportable events. A number of approaches can be used to reduce the risk of facial skin breakdown during NIV (Table 5).115 Perhaps the most important approach to prevent skin breakdown is to avoid strapping the mask too tight. A mask that is too large or that is too small increases the likelihood of poor fit and facial soreness. A mask with a forehead spacer or an adjustable forehead arm can be used to reduce the pressure on the bridge of the nose. Tape can be applied to the bridge of the nose, but this is less effective after substantial skin breakdown has occurred. Commercially available material is available specifically for this purpose. One can also consider the use of a different interface.
How to Reduce the Risk of Skin Damage During Noninvasive Ventilation
A total face mask creates a soft seal around the perimeter of the face, so there is no pressure on areas that nasal of oronasal masks contact. In subjects with acute respiratory failure, Ozsancak et al125 found that the oronasal mask and total face mask were perceived to be equally comfortable and had similar application times. In another study of subjects with acute respiratory failure, Chacur et al126 reported that the total face mask was more comfortable than the oronasal mask and suggested that the total face mask should be available as an option in units where NIV is routinely applied. In a normal volunteer study, Holanda et al127 found that the total face mask avoided pain on the bridge of the nose and presented no air leaks around the eyes and mouth. Belchior et al128 reported that the total face mask was very well tolerated by subjects who previously developed facial skin breakdown with an oronasal mask.
The helmet has a transparent hood and soft collar that seals at the neck.115 The helmet has 2 ports, one through which gas enters and another from which gas exits, and it is secured to the patient by armpit straps. The United States Food and Drug Administration has not cleared any of the available helmets, but they have been approved in some other countries, and they are popular at some places in Europe and South America. The helmet has a volume that is larger than the tidal volume, such that it behaves as a semi-closed environment in which the increase in inspired partial pressure of CO2 is an important issue. Inspired PCO2 in a helmet depends on the amount of CO2 exhaled by the patient and the fresh gas flow that flushes the helmet.129 High gas flow (40–60 L/min) is required to maintain a low inspired partial pressure of CO2. When compared to an oronasal mask, Racca et al130 found that use of the helmet to deliver PSV increased inspiratory muscle effort and asynchrony, worsened CO2 clearance, and increased dyspnea. Costa et al131 compared synchrony with invasive ventilation (endotracheal tube) and NIV with an oronasal mask or helmet as the interface. They found that patient-ventilator synchrony was significantly better with the endotracheal tube than with the mask or helmet. They also found that the helmet resulted in worse synchrony. An optimized set-up for helmet NIV that limits device compliance and ventilator circuit resistance as much as possible may be effective in improving pressure support delivery and patient-ventilator interaction.132
For applications of NIV for acute respiratory failure, the first choice of interface should be the oronasal mask. The available evidence suggests that the total face mask might also be a reasonable first choice for interface. Other interfaces should be available if the patient is intolerant of the oronasal mask or total face mask, or if complications such as facial skin breakdown occurs. Results of surveys in the United States133 and Europe134 have shown that clinicians most commonly favor the use of oronasal masks for NIV in patients with acute respiratory failure.
Which Ventilator?
Table 6 lists considerations in the selection of a ventilator for NIV.112,113,135 In North America, bi-level ventilators are commonly used for this purpose. They use a single limb circuit with a leak port, which serves as a passive exhalation port for the patient (Fig. 6).136 A leak port is incorporated into the circuit near the patient or in the interface. Although there is a potential for rebreathing with circuits that use a passive exhalation port, this is less likely with current generation designs in which the flow is adequate to flush the circuit of CO2. A blower generates inspiratory and expiratory pressures. Bi-level ventilators typically provide PSV or pressure control ventilation. Intermediate ventilators are commonly used for patient transport or home care ventilation. Many use a single limb circuit with an active exhalation valve near the patient, although some use a passive leak port similar to bi-level devices. Newer generations of intermediate ventilators provide volume-controlled, pressure-controlled, and PSV. Some newer generation bi-level and intermediate ventilators also provide adaptive pressure ventilation. Critical care ventilators have traditionally been designed for invasive ventilation, but newer generations have modes for NIV. For critical care ventilators, dual limb circuits are used and these have inspiratory and expiratory valves, and separate hoses for the inspiratory gas and the expiratory gas.
Considerations in the Selection of a Ventilator for Noninvasive Ventilation
Circuit configurations for noninvasive ventilation.
Several recent studies have evaluated the ability of critical care ventilators to compensate for leaks. In a bench study, Vignaux et al137 found that leaks interfere with the function of ICU ventilators, and that NIV modes can correct this problem, but with wide variations between ventilators. In a follow-up clinical study, Vignaux et al138 reported that NIV modes on ICU ventilators decreased the incidence of asynchrony typically associated with leaks. However, there was no change in overall asynchrony, perhaps because the correction of one asynchrony leads to an increase in another. In a bench study, Ferreira et al139 found that, in the presence of leaks, most ICU ventilators, but not all, required adjustments to maintain synchrony. In a laboratory and clinical study, Carteaux et al140 suggested that, as a group, bi-level ventilators outperform critical care ventilators for NIV. However, the NIV modes on some, but not all, critical care ventilators improve synchrony in the presence of leaks. Some critical care ventilators also allow clinicians to make adjustments to improve synchrony. These embellishments include an adjustable trigger type and sensitivity, an adjustable flow cycle criteria with PSV, and a maximal inspiratory time during PSV. Due to the differences in ability to compensate for leaks among ventilators used for NIV, it is important for clinicians to appreciate the unique characteristics of the ventilators they use.141
Due to the intentional leak port associated with the passive circuits used with bi-level ventilators, concern has been raised for the potential of exposure of healthcare workers to contaminants in the exhaled gas of the patient. Bench studies have reported substantial exposure to exhaled air within 1 m from patients receiving NIV in an isolation room with negative pressure.142,143 Thus, appropriate precautions are necessary when NIV is used for patients with highly contagious respiratory infections.
PSV is used most commonly for NIV applications in patients with acute respiratory failure. With a critical care ventilator the level of PSV is applied as a pressure above the baseline PEEP. However, the approach is different with bi-level ventilators, where an inspiratory positive airway pressure and expiratory airway pressure are set. In this configuration, the difference between the inspiratory and expiratory airway pressure is the level of PSV.
Proportional assist ventilation (PAV) and neurally adjusted ventilatory assist (NAVA) are modes intended to improve patient-ventilator synchrony.144 For PAV, Gay et al145 reported better patient tolerance with PAV, compared to PSV, during NIV. In the United States, the Food and Drug Administration has not cleared any ventilators for use of PAV for NIV. NAVA has been reported to improve synchrony during NIV when a helmet is used.146,147 Using an oronasal mask, Schmidt et al148 reported that NAVA improved synchrony more than the use of NIV mode on a critical care ventilator. The combination of NAVA with the NIV mode seemed to offer the best compromise between good synchrony and a low level of leaks. They also found a high level of leaks with NAVA, probably as a result of the nasogastric tube. The need for a specialized nasogastric tube is an important barrier to the use of NAVA. Average volume-assured pressure support is a form of adaptive pressure ventilation. With average volume-assured pressure support there is concern that the ventilator decreases support if respiratory drive increases. It is unclear whether the use of these newer modes improves outcomes in patients receiving NIV for acute respiratory failure.
How to Address Asynchrony?
The NIV failure rate (need for intubation) may be as high as 40%. Some of these failures may relate to asynchrony. Good NIV tolerance has been associated with success of NIV, and improved comfort has been associated with better synchrony. In one study, a high rate of asynchrony occurred in 43% of subjects during NIV.149 Patient-ventilator asynchrony during NIV is related to the underlying disease process and the presence of leaks.122 Thus, reducing the leak related to the interface and using a ventilator with good leak compensation should reduce the rate of asynchrony.
Is Humidification Necessary During NIV?
Whether or not humidification is necessary during NIV is controversial.150 In the presence of mouth leak with a nasal interface, unidirectional flow dries the upper airway and increases nasal airway resistance. Upper airway drying contributes to discomfort and may affect tolerance of NIV.151 Although anecdotal, my personal experience has been that heated humidification improves comfort and tolerance of NIV, and results in less upper airway drying. The level of humidification does not need to be as great as that for an intubated patient; 100% relative humidity at about 30°C is usually sufficient, and higher temperatures may be less comfortable during NIV. A heat and moisture exchanger is not recommended for use with NIV, because the additional dead space decreases carbon dioxide elimination, particularly in patients with hypercapnia.152
Can Inhaled Aerosols Be Delivered During NIV?
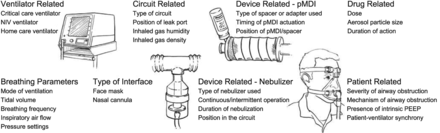
Patients with obstructive lung disease who are receiving NIV might also benefit from inhaled bronchodilator therapy. Aerosol therapy in this setting can be delivered effectively by pressurized metered-dose inhaler with a spacer or nebulizer.153,154 Alternatively, the patient can be removed from NIV and the inhaled medication administered in the usual manner,155 but this has the disadvantage of interrupting NIV. A number of factors affect aerosol delivery during NIV, and these include the type of ventilator, mode of ventilation, circuit conditions, type of interface, type of aerosol generator, drug-related factors, breathing parameters, and patient-related factors (Fig. 7). When a critical care ventilator is used for NIV, factors affecting aerosol delivery are much the same as the factors affecting aerosol delivery with invasive ventilation.156 Despite the impediments to efficient aerosol delivery with a bi-level ventilator, due to the continuous gas flow and leaks, substantial therapeutic effects are achieved after inhaled bronchodilator administration to patients with asthma and COPD. Galindo-Filho and colleagues157 reported that, although coupling nebulization and NIV during an asthma exacerbation did not improve radio-aerosol pulmonary deposition, it did result in clinical improvement of pulmonary function in these patients. Careful attention to the technique of drug administration is required to optimize therapeutic effects of inhaled drugs during NIV.
Factors influencing aerosol delivery during noninvasive ventilation (NIV). pMDI = pressurized metered-dose inhaler. (From Reference 153, with permission.)
Should NIV Be Used With Heliox?
The evidence for the use of heliox in patients with COPD exacerbation is weak.158 Most of the peer-reviewed literature consists of case reports, case series, and physiologic studies in small samples of carefully selected patients. Some patients with COPD exacerbation have a favorable physiologic response to heliox therapy, but predicting who will respond is difficult. Maggiore et al159 assessed the effect of heliox on intubation rate and clinical outcomes during NIV in subjects with COPD exacerbation. NIV was randomly applied with or without heliox. Intubation rate did not significantly differ between groups, and there was no difference observed in blood gases, dyspnea, or breathing frequency between groups. The available evidence does not support the use of heliox in patients with COPD exacerbation; it certainly cannot be considered standard therapy.160 If heliox is used in conjunction with NIV, the effect of heliox on ventilator function must also be considered.161
Complications of Noninvasive Ventilation
Complications from NIV are usually minor, including mask discomfort, mild asynchrony due to leaks, upper airway discomfort due to inadequate humidification, and mild gastric insufflation. More serious complications include facial skin breakdown, gastric distention, regurgitation and aspiration, and the hemodynamic effects of the positive intrathoracic pressure. Serious complications due to NIV are thought to be infrequent, but this has not been systematically evaluated. An issue of concern is inappropriate use of NIV for too long when the therapy is failing, which may increase mortality due to excessive delay of intubation. Clinicians should be aware of potential complications of NIV and regularly assess patients to minimize these complications.162
NIV, Ventilator-Associated Pneumonia, and Ventilator-Associated Events
It is recognized that the source of VAP is usually micro-aspiration of upper airway secretions from above the cuff of the endotracheal tube. Thus, avoidance of invasive ventilation (eg, NIV) should decrease the risk of VAP.163 Indeed, several meta-analyses have reported lower VAP rates with the use of NIV.24,164 In the United States, surveillance of ventilator-associated events began in 2013. A ventilator-associated event is triggered by a sustained increase in FIO2 or PEEP after a period of stability while receiving invasive ventilation. Thus, the use of NIV to prevent intubation or to allow earlier extubation should decrease the risk of a ventilator-associated event (http://www.cdc.gov/nhsn/acute-care-hospital/vae/index.html).
How to Improve Utilization
NIV is underutilized, despite the robust evidence reviewed in this paper.133,165–167 Increased utilization requires that clinicians view it as often superior to invasive ventilation, that it is perceived as compatible with existing approaches to mechanical ventilation, and that it is not too difficult to apply.112,168 Barriers to NIV use include lack of awareness of the evidence, lack of agreement with the evidence, lack of self-efficacy, unrealistic outcome expectations, and the inertia of previous practice. A clinical champion is important when initiating and expanding an NIV program. Knowledge and training are also important, with one-on-one and hands-on practice to the extent possible. Adequate personnel and equipment resources are necessary when implementing the program. Guidelines and protocols may be useful as educational resources.169–171 When initiating an NIV program, it is important to recognize that NIV does not avoid intubation in all cases, and that success often improves with experience.98 The available evidence suggests that NIV is cost-effective.172,173 For optimum success the multidisciplinary nature of NIV application must be recognized.
Summary
Substantial evidence supports the use of NIV in appropriately selected patients. For patients presenting with COPD exacerbation or acute cardiogenic pulmonary edema, use of NIV is considered standard practice. NIV should be part of the armamentarium of all clinicians caring from patients with acute respiratory failure.
Footnotes
- Correspondence: Dean R Hess PhD RRT FAARC, Respiratory Care, Ellison 401, Massachusetts General Hospital, 55 Fruit Street, Boston MA 02114. E-mail: dhess{at}partners.org.
Dr Hess presented a version of this paper at the 51st Respiratory Care Journal Conference, “Adult Mechanical Ventilation in Acute Care: Issues and Controversies,” held September 7 and 8, 2012, in St Petersburg, Florida.
Dr Hess has disclosed relationships with Philips Respironics, ResMed, Pari, Breathe, Covidien, and Maquet.
- Copyright © 2013 by Daedalus Enterprises
References
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.
- 15.
- 16.
- 17.
- 18.
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- 64.↵
- 65.↵
- 66.↵
- 67.
- 68.
- 69.↵
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.↵
- 75.↵
- 76.↵
- 77.
- 78.↵
- 79.
- 80.
- 81.↵
- 82.↵
- 83.↵
- 84.↵
- 85.↵
- 86.↵
- 87.↵
- 88.↵
- 89.
- 90.
- 91.
- 92.
- 93.
- 94.
- 95.
- 96.↵
- 97.↵
- 98.↵
- 99.↵
- 100.↵
- 101.↵
- 102.↵
- 103.↵
- 104.↵
- 105.↵
- 106.↵
- 107.↵
- 108.↵
- 109.↵
- 110.↵
- 111.↵
- 112.↵
- 113.↵
- 114.↵
- 115.↵
- 116.↵
- 117.↵
- 118.↵
- 119.↵
- 120.↵
- 121.↵
- 122.↵
- 123.↵
- 124.↵
- 125.↵
- 126.↵
- 127.↵
- 128.↵
- 129.↵
- 130.↵
- 131.↵
- 132.↵
- 133.↵
- 134.↵
- 135.↵
- 136.↵
- 137.↵
- 138.↵
- 139.↵
- 140.↵
- 141.↵
- 142.↵
- 143.↵
- 144.↵
- 145.↵
- 146.↵
- 147.↵
- 148.↵
- 149.↵
- 150.↵
- 151.↵
- 152.↵
- 153.↵
- 154.↵
- 155.↵
- 156.↵
- 157.↵
- 158.↵
- 159.↵
- 160.↵
- 161.↵
- 162.↵
- 163.↵
- 164.↵
- 165.↵
- 166.
- 167.↵
- 168.↵
- 169.↵
- 170.
- 171.↵
- 172.↵
- 173.↵
Discussion
Berra:
Cooperation between all the healthcare providers is essential to successfully implement NIV in clinical practice. In my experience, ICU staff unfamiliarity with NIV support and devices is a major obstacle when introducing NIV, and education projects may be helpful. Sometimes it may be difficult to apply the results of these impressive studies in everyday clinical practice.
Hess:
I think that the success of NIV depends first upon selecting the right patient, as I talked about that a lot in this presentation. Second, you have to choose the right equipment. There are different types of interfaces, different ventilators, and different ventilator settings. Third, and perhaps more important than the previous two, are the skills of the clinician, ideally a clinician who is skillful at adapting this therapy to the patient at the bedside.
Schmidt:
I think it's not just a question of skill, but also of optimizing time and value in the unit. Starting someone on NIV takes an hour or two of the therapist's time. If you have a busy unit with 20 patients intubated and you have to run around transporting patients, sometimes there might not be enough time to justify the benefit for a particular patient, at the risk of not providing the best care for other patients in the ICU. It might be better to just intubate. In my opinion, the resource allocation with NIV is sometimes challenging.
Kacmarek:
I'm sorry to disagree with my Medical Director, but I will, because I think it's a matter of setting priorities and getting the support to make it happen. There's really no reason that we—and I'll speak for Massachusetts General Hospital—should not be able to allocate the time to start a patient on NIV, because it's clearly to the patient's benefit if we can manage them noninvasively. I agree 100% with Lorenzo and Dean that the individual clinician can make a huge difference as to whether NIV is successful. NIV should not be failing because of the therapist. I can't say that it doesn't happen, but it shouldn't be happening. Every one of us should try to have mechanisms to accommodate busy circumstances and bring additional therapists to make NIV successful, if at all possible.
Hess:
So, along with skills of the clinicians, maybe we need to say skills and biases of the clinicians?
Marini:
I have two observations that impress me as an intensive care practitioner. One is that NIV post-extubation—or even pre-intubation—is often set up without humidification, because it's easier and faster for the therapist. Breathing through an open mouth with a high FIO2 in Boston or Minnesota in the wintertime, it's very dry. Secretion thickening becomes an important issue after extubation. And during the immediate post-extubation period I use NIV, especially at night. These patients have residual sedation and they may be predisposed to OSA [obstructive sleep apnea], and fluid shifts are prevalent. It's really important for the post-extubation caregivers to think about those 2 issues: use NIV liberally at night, and add hydration. Do you agree with that?
Hess:
Absolutely. A couple of thoughts. First, on humidification: I think it's very important during NIV, and in fact at Massachusetts General Hospital it is standard practice to always deliver humidity. That comes out of some anecdotal experiences that we had where patients were failing NIV, and when the time came to intubate, large amounts of dried secretions were removed and intubation was avoided. So I think humidity is very important. I have a section of my paper on that subject. There's some evidence on using NIV in acute respiratory failure, and there's a lot of evidence related to humidification that we can extrapolate from patients who use nocturnal CPAP for OSA. I do like the fact that you brought up the potential for OSA being the cause of respiratory failure post-extubation. We don't think nearly enough about that. In the patient who is extubated in the morning and then has a big desaturation and is reintubated the following night: I wonder if they had undiagnosed OSA and just needed CPAP.
Marini:
These are the perfect conditions to bring it out. Anybody who's had an extra beer at night knows that they snore more under the influence of alcohol. We give these people a lot of drugs that are still in their system, they may be weak, and they may be sleep-deprived. The first time they can go deeply to sleep it will uncover OSA. Even if they didn't have it as an out-patient, they might have it then.
Hess:
I agree. We don't do a very good job recognizing it.
Blakeman:
Rich Branson and I work primarily in surgical trauma, so the patients use NIV mostly for hypoxemic respiratory failure, and we know from your talk that there's no mortality difference between the groups. Anecdotally, we have some intensivists who would like to put them on NIV for days, even though they're on that flat slope most of the time. We found that in that group they died more often than those who did not receive it or did not receive it as long. So, at least anecdotally, in our experience, there's been a direct correlation that if you leave them on too long, you can actually hurt the patient.
Hess:
I think there is evidence to support that, which goes back to assessing these patients after 1 or 2 hours, and then deciding whether they are getting better, or they are not and you stop.
Blakeman:
We've tried to mandate that with our attendings. After a couple hours, if the patient doesn't improve, we intubate.
Branson:
Dean, it's not FDA cleared, but what do you think about the NIV helmet? I'm underwhelmed by it. I've never put one on a patient, obviously, since it's not FDA cleared. I have worn one, but that's the extent of my experience.
Hess:
There are several issues with the helmet, and Lorenzo can chime in since he is from Italy, the country that is the biggest helmet-user. I have two issues with the helmet, one is that you need to have enough flow through the device to clear out CO2. Our group published a paper in Critical Care Medicine showing that there can be substantial CO2 accumulation within the helmet if the flow is not great enough.1 You could say, well, maybe it's OK for CPAP, and in fact that's how they used it in the Squadrone study.2 The second issue that I have is how it impacts the ability of the patient to trigger and cycle the ventilator on pressure support. There was a very nice paper by Ranieri's group in the Journal of Applied Physiology, showing that there can be big issues with triggering and cycling inside the helmet.3 Lorenzo, I look at you because you have the entirety of this room's experience with the helmet.
Berra:
One advantage of the helmet is greater patient comfort, especially outside the ICU setting.
Hess:
But many of these are CPAP and cardiogenic pulmonary edema.
Berra:
Yes, yet CPAP may be a good option in different challenging situations, from transport of critically ill patients between facilities to awake patients on ECMO [extracorporeal membrane oxygenation] for respiratory failure.
Kacmarek:
In ARDS patients on ECMO it was for CPAP, and not for ventilation?
Berra:
Yes, they are awake and spontaneously breathing on CPAP.
Kacmarek:
And you use a continuous flow?
Berra:
Yes.
Gajic:
I have a question about humidified high-flow nasal cannula, which has been creeping up in our practice. We tend to use it in patients with hypoxemic respiratory failure or post-extubation. We found it less resource intensive and well tolerated, compared to NIV. I'm interested in your thoughts.
Hess:
There has been a lot published in Respiratory Care and other journals just in the past few years on humidified high-flow O2 therapy. One of the things that is unclear is, what is the mechanism of benefit? One benefit might be that, instead of having a face mask on the patient, we are using a nasal cannula, so there's no intermittent being off of O2 when the patient removes the mask to cough or take medicine or eat and drink, and so forth. There have been several studies that have suggested that patient comfort and tolerance is better with the high-flow nasal cannula than with face mask, so maybe one of the benefits is that it is more comfortable for the patient so they are more tolerant of the therapy.
Then there is the question of whether it has a CPAP effect, because you're blowing 40 L/min of gas into the pharynx and that opposes exhalation and produces some CPAP. There is probably a little bit, but maybe not enough that it has that much therapeutic benefit.
We published a paper in Respiratory Care where the group looked at pharyngeal pressures using a high-flow nasal cannula—these were adult patients—and they found that there were a few cm H2O of pharyngeal pressure at end exhalation (CPAP) if the patient kept their mouth closed.4 But as soon as they opened their mouth, the CPAP effect went away.
Then there is another potential mechanism that might be important in patients with COPD, which is that the high flow flushes CO2 from the upper airway and in that way decreases the ventilatory requirement for the patient, which is something that some of us were interested in 15 years ago, and we called it tracheal gas insufflation. There may be some of that effect with the use of high-flow nasal cannula.
Kallet:
I think there appears to be a lot of excess enthusiasm about high-flow nasal cannula. I've had situations where clinicians are talking physicians out of ordering mask CPAP or NIV in patients with COPD or cardiogenic pulmonary edema. Whether there's strong evidence saying it's equivalent—it's the problem of stuff being hyped without evidence to back it up. In this case there is very clear evidence backing mask CPAP and NIV in the populations Dean talked about. We should not be advocating so vociferously for high-flow nasal cannula in these circumstances, unless someone is not tolerating NIV. High-flow cannula is probably more comfortable, but it shouldn't be the first choice. It's clearly indicated that patients with cardiogenic pulmonary edema and COPD should be managed with NIV.
Hess:
I know of no RCTs—a lot of observational studies, but no RCTs of NIV or CPAP versus high-flow nasal cannula.
Gajic:
That's why I said outside of COPD exacerbations, specifically for that purpose. That's a very good point.
Turner:
In pediatrics, in many patients we can't use NIV because the interfaces do not work well, and this has probably contributed to the enthusiasm for high-flow nasal cannula in pediatrics. While the data are limited, there may be clinical benefit in select circumstances.