Abstract
BACKGROUND: Awake prone positioning (APP) has been recently proposed as an adjunctive treatment for non-intubated coronavirus disease 2019 (COVID-19) patients requiring oxygen therapy to improve oxygenation and reduce the risk of intubation. However, the magnitude of the effect of APP on clinical outcomes in these patients remains uncertain. We performed a comparative systematic review and meta-analysis to evaluate the effectiveness of APP to improve the clinical outcomes in non-intubated subjects with COVID-19.
METHODS: The primary outcomes were the need for endotracheal intubation and mortality. The secondary outcome was hospital length of stay. Pooled risk ratio (RR) and mean difference with the corresponding 95% CI were obtained by the Mantel-Haenszel method within a random-effect model.
RESULTS: A total of 14 studies (5 randomized controlled trials [RCTs] and 9 observational studies) involving 3,324 subjects (1,495 received APP and 1,829 did not) were included. There was a significant reduction in the mortality rate in APP group compared to control (RR 0.68 [95% CI 0.51–0.90]; P = .008, I2 = 52%) with no significant effect on intubation (RR 0.85 [95% CI 0.66–1.08]; P = .17, I2 = 63%) or hospital length of stay (mean difference −3.09 d [95% CI−10.14–3.96]; P = .39, I2 = 97%). Subgroup analysis of RCTs showed significant reduction in intubation rate (RR 0.83 [95% CI 0.72–0.97]; P = .02, I2 = 0%).
CONCLUSIONS: APP has the potential to reduce the in-hospital mortality rate in COVID-19 subjects with hypoxemia without a significant effect on the need for intubation or length of hospital stay. However, there was a significant decrease in the need for intubation on subgroup analysis of RCTs. More large-scale trials with a standardized protocol for prone positioning are needed to better evaluate its effectiveness in this select population.
Introduction
Coronavirus disease 2019 (COVID-19) due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has led to significant morbidity and mortality globally.1 ARDS occurs in 20–41% of patients with severe COVID-19.2 Prone positioning is known to improve oxygenation and mortality in mechanically ventilated and intubated patients with moderate-to-severe ARDS.3 Prone positioning improves oxygenation by reducing ventilation/perfusion mismatching and reducing intrapulmonary shunt.4
Utilizing awake prone positioning (APP) has been recently proposed as an adjunctive treatment for spontaneously breathing non-intubated COVID-19 patients requiring oxygen therapy to reduce the risk of intubation.5 Most published studies showed significant improvement in oxygenation parameters such as PaO2/FIO2, PaO2, and SpO2 after APP sessions.4-6 Several single-arm meta-analyses have evaluated the effect of APP on oxygenation parameters and pooled the overall mortality and intubation rates in subjects who underwent APP without a control group.7 However, the magnitude of the effect of APP on clinical outcomes (eg, the risk of endotracheal intubation or mortality) in subjects with COVID-19 remains uncertain. Therefore, we performed a comparative meta-analysis to evaluate the effectiveness of APP to improve the clinical outcomes in subjects with COVID-19.
Methods
Data Sources and Search Strategy
We performed a comprehensive search for published studies indexed in PubMed/MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials from inception to August 30, 2021. We also performed a manual search for additional relevant studies using references of the included articles. The following search terms were used: (“prone positioning” or “prone position”) and (“COVID-19” or “SARS-CoV-2”). The search was not limited by language, study design, or country of origin. Supplementary Table 1 (see related supplementary materials at http://www.rc.rcjournal.com) describes the full search term used in each database searched.
Inclusion and Exclusion Criteria
All published studies (randomized controlled trials [RCTs] and observational studies) that compared APP versus a control group in non-intubated COVID-19 subjects and reported one of the following outcomes: endotracheal intubation, mortality, or length of hospital stay, were eligible for inclusion. All the studies that did not report endotracheal intubation or mortality rates were excluded, such as a study by Kharat et al.8 Only adult subjects age 18 y or older were eligible for inclusion. We excluded single-arm studies, case reports, reviews, commentaries, preprints (not peer reviewed), and abstracts.
Data Extraction
The following data were extracted from the studies: first-author name, publication year, country of origin, study design, number of subjects, follow-up duration, subject location, outcomes measures including rates of intubation, mortality, and length of hospital stay. We also extracted details of APP; oxygen and noninvasive respiratory support; and subjects’ baseline comorbidities, including body mass index, hypertension, diabetes mellitus, and COPD. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement guidelines to select the final studies. Two investigators (MM and AB) independently performed the search and short-listed the studies for final review. Discrepancies were resolved by a third reviewer (OS). We did not contact the authors of the studies.
Outcomes
The primary outcomes of our study were the need for endotracheal intubation and mortality between the APP and control groups of non-intubated subjects with COVID-19. The secondary outcome was the length of hospital stay between the 2 groups.
Statistical Analysis
We performed a meta-analysis of the included studies using Review Manager 5.3 (Cochrane, London, United Kingdom) and Comprehensive Meta-Analysis (Biostat, Englewood, New Jersey). The median and interquartile range were converted to mean and SD where applicable.9 The random-effects model was used to calculate the pooled risk ratio (RR) and mean difference with the corresponding CI for proportional and continuous variables, respectively. A P value < .05 was considered statistically significant. The heterogeneity of the effect size estimates across the studies was quantified using the Q statistic and I2 (P < .10 was considered significant). A value of I2 of 0–25% indicates insignificant heterogeneity, 26–50% low heterogeneity, 51–75% moderate heterogeneity, and 76–100% high heterogeneity.10
Subgroup and Sensitivity Analyses for Endotracheal Intubation and Mortality
We performed a subgroup analysis of RCTs for endotracheal intubation. To confirm the robustness of the results, sensitivity analysis for endotracheal intubation and mortality using leave-one-out meta-analysis was performed to see if it had a significant influence on the result of the meta-analysis.
Bias Assessment
The Jadad composite scale was used to assess the methodological quality of the RCTs based on randomization, blinding, and withdrawals.11 The scale ranged from 0–5 points.12 Studies with a total score > 3 were considered high quality, whereas moderate quality if a score of 3, and low quality if < 3. The Newcastle-Ottawa Quality Assessment Scale was used to assess the quality of the observational studies based on the selection of the study groups, comparability of study groups, and ascertainment of exposure/outcome.12 Studies with a total score > 6 were considered high quality, whereas moderate quality if a score of 6, and low quality if < 6. Publication bias was assessed qualitatively by visually assessing the funnel plot and quantitively using Egger regression analysis. Two authors (AB and MM) independently assessed each study for bias. Discrepancies were resolved by a third reviewer (OS).
Results
Study Selection
A total of 1,048 studies were retrieved by our search strategy. Among these, 57 were eligible for systematic review. Subsequently, we excluded 44 studies because of subjects were intubated, absence of control group, lack of appropriate outcome, or lack of appropriate control. Eventually, 14 studies5,6,13-24 met our inclusion criteria and were included in the meta-analysis. Figure 1 shows the PRISMA flow chart that illustrates how the final studies were selected.
Flow chart.
Quality and Publication Bias Assessment
Quality assessment scores of the RCTs and observational studies are summarized in Supplementary Table 2 (see related supplementary materials at http://www.rc.rcjournal.com). All the included studies were of moderate or high quality. Four RCTs6,20,22,23 were of moderate quality, and one RCT17 was of high quality. Six observational studies5,13,15,16,19,21 had a total score > 6, indicating that they were of high quality. Three observational studies14,18,24 scored 6, indicating that they were of moderate quality.
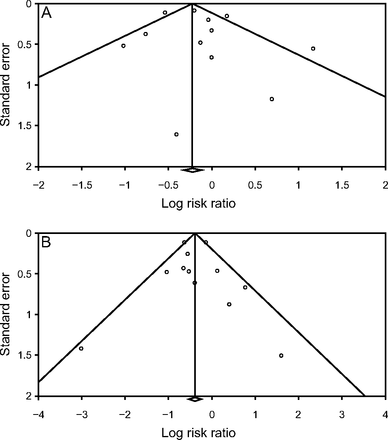
There was a visible asymmetry in the funnel plot of the studies that reported both the need for endotracheal intubation and in-hospital mortality, which may suggest the presence of publication bias (Fig. 4). However, Egger regression analysis did not demonstrate statistically significant publication bias (P = .54 and P = .91 for endotracheal intubation and mortality, respectively).
Study and Subjects’ Characteristics
Tables 1 and 2 show the study and subject characteristics of the studies included in the meta-analysis. All the included studies were published between 2020–2021 and included spontaneously breathing non-intubated subjects with laboratory-confirmed COVID-19. Based on country of origin, 3 studies originated from the United States; 5 studies originated from Europe (Italy, France, Spain, and Sweden), 3 studies from Asia (China and India), 2 studies from South America (Brazil, Mexico, and Ecuador), and one multinational study (meta-trial from 6 countries: Canada, Ireland, France, Mexico, Spain, and United States. Regarding the design of included studies, 5 were RCTs and 9 were observational cohort studies (6 studies were retrospective cohort, and 3 were prospective cohort).
Characteristics of the Included Studies
Subject Characteristics and Outcomes of the Included Studies in the Meta-Analysis
A total of 3,324 subjects (1,495 received APP and 1,829 did not) were included, with males representing 69.8% of the total subjects. The follow-up period across the studies ranged from 14–90 d. The assessment of the risk of bias is presented in Supplementary Table 2. All 9 observational studies scored ≥ 6 on the Newcastle-Ottawa Scale, and all 5 RCTs scored ≥ 3, representing a low risk of bias (see related supplementary materials at http://rc.rcjournal.com).
Outcomes
Need for endotracheal intubation.
Table 2 summarizes the outcomes of the individual studies included in the meta-analysis. Across the 13 studies5,6,13-20,22-24 that reported the intubation rate, 27% of subjects who received APP required intubation compared to 29.8% in subjects who did not receive APP. The need for endotracheal intubation was similar between APP and control groups (RR 0.85 [95% CI 0.66–1.08], P = .17); the statistical heterogeneity was moderate with I2 of 63% (Fig. 2A). However, on subgroup analysis of RCTs, the need for intubation was significantly reduced in the APP group versus control (RR 0.83 [95% CI 0.72–0.97]; P = .02, I2 = 0%) (Fig. 3). To assess the stability of the results of our meta-analyses, we performed a one-study removed sensitivity analysis. Removal of Zang et al, moved the overall effect to favor APP (RR 0.80 [95% CI: 0.64–0.99]), suggesting that Zang et al19 was partly the reason for the moderate between-study heterogeneity. (Supplementary Fig. 1, see related supplementary materials at http://www.rc.rcjournal.com).
Forest plots comparing awake prone positioning and control regarding A: endotracheal intubation, B: mortality, and C: hospital stay. APP = awake prone positioning.
Subgroup analysis of randomized controlled trials for the need for endotracheal intubation. APP = awake prone positioning.
Funnel plots comparing awake prone positioning and control regarding A: endotracheal intubation and B: mortality.
Mortality.
Thirteen studies5,6,13,14,16-24 reported the mortality rate. The mortality rate was 17.9% in the APP group compared to 25.7% in the control group. There was a statistically significant difference in the mortality rate between the 2 groups (RR 0.68 [95% CI 0.51–0.90], P = .008), and the statistical heterogeneity was moderate with I2 of 52% (Fig. 2B). A leave-one-out sensitivity analysis showed consistent results (Supplementary Fig. 2, see related supplementary materials at http://www.rc.rcjournal.com).
Discussion
Prone positioning for treating ARDS is a well-known strategy to improve oxygenation; it is recommended for 16 h daily in mechanically ventilated and intubated patients with ARDS with PaO2/FIO2 < 150 mm Hg.3 However, the evidence regarding the utility of APP in non-intubated patients is limited.
In the current COVID-19 pandemic era, a substantial number of COVID-19 patients developed ARDS, with an extreme surge in need for respiratory support, intubation, and mechanical ventilation. Hence, every effort was implemented to avoid intubation and mechanical ventilation given the shortage of human and medical resources.25 One of the proposed strategies to avoid such complications is to implement prone positioning in awake, spontaneously breathing patients in the hope of preventing further respiratory deterioration and the need for advanced respiratory support.26
Several studies have shown significant improvement in oxygenation parameters such as PaO2/FIO2, PaO2, SpO2, and breathing frequency.27-29 Some meta-analyses have investigated the effect of APP on clinical outcomes in non-intubated subjects with COVID-19 by pooling the rates of intubation and mortality from single-arm studies that did not have a control group.7,30 A meta-analysis by Pavlov et al30 has pooled the mortality and intubation rates in single-arm studies and showed the pooled intubation rate of 27% among those who underwent APP. However, few studies have evaluated the effects of APP versus control on clinical outcomes of patients with COVID-19.13,14,18,19 To our knowledge, only one comparative meta-analysis by Chua et al31 has been published that evaluated the impact of APP versus control on intubation and mortality rates in subjects with COVID-19. However, Chua et al31 included a limited number of studies (5 studies), and all the included studies were observational (no RCT). Further studies, including RCTs,6,20,23 have been published; and we provide an updated comparative meta-analysis to investigate the effect of APP compared to control on the clinical outcomes, including the need for intubation, in-hospital mortality, and hospital length of stay.
Our meta-analysis demonstrated that APP reduced mortality in non-intubated COVID-19 subjects without a significant difference in the need for endotracheal intubation and length of hospital stay. However, APP showed a significant reduction in the need for intubation when subgroup analysis was restricted to RCTs.
Our meta-analysis results are similar to those from the recent meta-analysis by Chua et al31 that revealed that APP could decrease mortality rate (odds ratio 0.35 [95% CI 0.16–0.75], P = .007) without significant effect on intubation rate (odds ratio 1.20 [95% CI 0.77–1.86], P = .42) in non-intubated COVID-19 subjects with hypoxia. However, we further conducted a subgroup analysis of RCTs for intubation, which significantly reduced the need for intubation. Furthermore, sensitivity analysis did not show consistent findings for intubation. This discrepancy might be attributed to the inconsistent protocols of APP utilized in the included studies and to the absence of standardized criteria and indications for intubation.32 Thus, we believe that there is an urgent need for guidelines and protocols to guide the practice of APP in COVID-19 patients. The protocols should include the eligibility criteria to initiate APP, number of sessions and average time per day, proper follow-up protocols, early identification of complications and treatment failure, and standardized criteria for intubation.
Our study has certain limitations. First, this meta-analysis was mainly based on observational studies and only included 5 RCTs. Therefore, more large-scale RCTs to investigate the impact of APP on the clinical outcomes of COVID‐19 subjects are warranted. Second, moderate heterogeneity was found in the measurement of intubation and mortality. This can be explained by inconsistent follow-up duration among the studies, significant variation in the respiratory support used, wide range of frequency and duration of the applied APP protocols, and significant difference in the location of the study (emergency department vs hospital ward vs ICU).32 Third, we were not able to assess the oxygenation parameters before and after APP sessions due to limited reported data. Fourth, the majority of the studies did not assess the APP protocol adherence, and there was a lack of standardized protocol and optimal duration for APP among studies. Lastly, the lack of patient-level data did not allow to control for possible variations in baseline characteristics or adoption of APP.
Despite the limitations, our study has significant strengths. First, we included a total of 14 studies with over 3,300 subjects with COVID-19. This is by far the largest analysis comparing the effect of APP on clinical outcomes in COVID-19 subjects with hypoxia. Although significant heterogeneity was noted, we performed a subgroup analysis of RCTs and sensitivity analysis to evaluate the robustness of our results. Consistent results were observed on sensitivity analysis for morality. Despite the presumed heterogeneity, the meta-analysis was undertaken to observe the effect of APP in subjects with COVID-19, as several studies revealed some benefits in terms of mortality and intubation rates. Finally, all the included studies were of moderate or high quality based on quality assessment.
Conclusions
APP has the potential to reduce the in-hospital mortality rate in COVID-19 patients with hypoxia without a significant effect on the need for intubation or length of hospital stay. However, there was a significant decrease in the need for intubation on subgroup analysis of RCTs. More large-scale trials with a standardized protocol for APP are needed to better evaluate its effectiveness in this select population.
Footnotes
- Correspondence: Azizullah Beran MD, Department of Internal Medicine, University of Toledo, 2100 W. Central Ave, Toledo, OH 43606. E-mail: Azizullah.Beran{at}utoledo.edu
Supplementary material related to this paper is available at http://rc.rcjournal.com.
The authors have disclosed no conflicts of interest.
- Copyright © 2022 by Daedalus Enterprises