Abstract
Acute respiratory failure with inadequate oxygenation and/or ventilation is a common reason for ICU admission in children and adults. Despite the morbidity and mortality associated with acute respiratory failure, few proven treatment options exist beyond invasive ventilation. Attempts to develop intravascular respiratory assist catheters capable of providing clinically important gas exchange have had limited success. Only one device, the IVOX catheter, was tested in human clinical trials before development was halted without FDA approval. Overcoming the technical challenges associated with providing safe and effective gas exchange within the confines of the intravascular space remains a daunting task for physicians and engineers. It requires a detailed understanding of the fundamentals of gas transport and respiratory physiology to optimize the design for a successful device. This article reviews the potential benefits of such respiratory assist catheters, considerations for device design, previous attempts at intravascular gas exchange, and the motivation for continued development efforts.
- respiratory assist catheter
- intravascular gas exchange
- intravascular oxygenator
- membrane oxygenation
- respiratory failure
- ARDS
- hypoxemia
Introduction
Acute respiratory failure with inadequate oxygenation and/or ventilation is a common reason for ICU admission in children and adults. Prior to the current COVID-19 pandemic, ARDS, a subset of hypoxic respiratory failure, was present in 7% of adult ICU patients and represented 1–10% of pediatric ICU admissions.1-4 The COVID-19 pandemic has only increased the burden of ARDS on ICUs.5,6 Despite the morbidity and mortality related to ARDS, few proven treatment options exist beyond invasive ventilation. When mechanical ventilation fails to adequately oxygenate these patients, one potential option is venovenous extracorporeal membrane oxygenation (VV-ECMO). ECMO is the only available therapy that directly oxygenates blood independent of the lungs and, therefore, is capable of fully supporting a patient regardless of degree of lung injury. However, ECMO is associated with potential complications, including hemorrhage, thrombosis, and infection.7 Further, ECMO is only available in approximately 3% of hospitals in the United States and fewer worldwide.8 The complexity and expense of ECMO, its associated morbidity, and its low availability limit the benefits of this potentially lifesaving technology. There is a need for alternative technologies that support patients with severe respiratory failure that function independently of diseased lungs.
In this light, novel therapies such as an intravascular gas exchange device are an attractive option. Ideally, an intravascular respiratory assist catheter capable of providing clinically important gas exchange would have a similar risk profile to a large central venous catheter and could be available to patients worldwide. Depending on efficiency and safety, a respiratory assist catheter could be used either independently or in conjunction with mechanical ventilation. This article reviews the potential benefits of intravascular respiratory assist catheters, considerations for device design, previous attempts at intravascular gas exchange, and the motivation for continued development efforts.
Description and Potential Benefits of Intravascular Gas Exchange Catheter
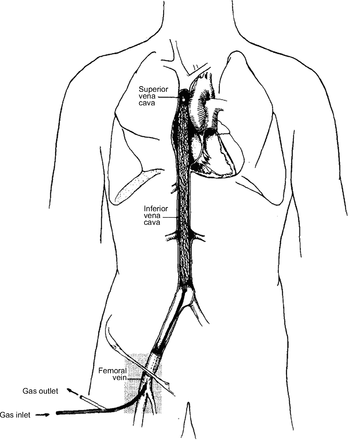
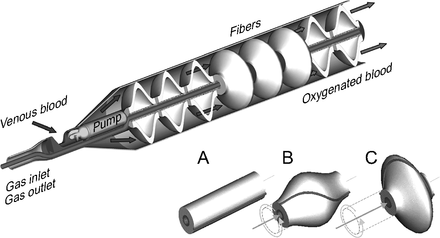
An intravascular respiratory assist catheter is a central venous catheter that participates in gas exchange to support patients with respiratory failure. The device is inserted into a central vein either percutaneously or with a venous cutdown (Fig. 1). These catheters could be placed at the bedside, in an ambulance, or even in the field. Ideally, once inserted, operation would involve basic titration of the gas flowing through the device. The benefits of an intravascular gas exchange catheter exist on a spectrum dependent on the efficiency of gas exchange coupled with its risk profile.
The IVOX catheter, an example of an intravascular respiratory assist catheter. This catheter is inserted via the femoral vein and lies within a patient’s vena cava. From Reference 32, with permission.
A complete respiratory assist catheter should be effective at both delivering O2 and removing CO2. Alternatively, there could be devices designed that predominantly deliver O2 or remove CO2 that would not be considered complete respiratory assist catheters. For lung disease with resultant hypoxic respiratory failure, an intravascular catheter that delivers O2 could be used independently or with supplemental O2/mechanical ventilation. When combined with mechanical ventilation, a respiratory assist catheter could allow for reduced ventilator support and thus less risk for ventilator-induced lung injury and pulmonary O2 toxicity. Hattler et al9 suggested that providing approximately 20% of an adult’s basal O2 needs (or about 50 mL O2 min−1) would achieve an arterial O2 saturation of 90% in patients with only 50% of their native lung function intact. Clinically, Conrad et al10 showed that when the IVOX catheter, the only intravascular respiratory assist catheter to be tested in a human clinical trial, delivered 40–70 mL O2 min−1 it allowed for a reduction of mechanical ventilation by at least 25% in approximately 50% of subjects.
In patients whose lung injury is associated with limited ventilation, a complete respiratory assist catheter, with the ability to remove CO2, could allow for decreased ventilator driving pressure (ΔP = VT/CRS), which Amato et al11 showed was strongly associated with decreased mortality in adults with ARDS. The practice of supporting patients with lung-protective strategies has shown survival benefits in randomized controlled clinical trials involving adults with ARDS.12-15 It is possible these survival benefits may extend to patients in whom respiratory assist catheters would allow for decreased ventilator support.
Patients with acute respiratory failure who remain hypoxic despite optimizing mechanical ventilation may also benefit from an intravascular respiratory assist catheter by reducing the need for VV-ECMO. As the risks of ECMO increase with the duration of extracorporeal support, shortening the total time a patient requires such support by augmenting oxygenation with a respiratory assist catheter could be beneficial. The smaller foreign surface area of an intravascular catheter (ranging from 0.0125–0.5 m2) in contact with the blood is an important advantage as it could allow for decreased systemic anticoagulation and may generate less of an inflammatory response when compared with the large surface area of an ECMO membrane oxygenator (1.8 m2).16 A catheter would not require the extracorporeal tubing nor housing for the hollow fiber membranes (HFMs) and, therefore, would not require a large crystalloid prime, avoiding hemodilution and possible fluid shifts in a patient with respiratory failure. This is a particularly important advantage in pediatrics as the ECMO circuit is large relative to an infant’s total blood volume and, therefore, requires the use of a blood component prime with the associated risks related to transfusion.17-19 Ideally, an intravascular oxygenator would not require an integrated pump, thereby avoiding an important source of hemolysis.20 Additionally, the risk of infection when a patient is supported with an intravascular oxygenator may be less than that of ECMO given that blood is not pumped through an extracorporeal circuit containing multiple joints and Luer-Lok access points. Finally, depending on the respiratory assist catheters’ configuration as it relates to ease of insertion and the required resources for operation, such technology may be a treatment option for patients in resource-limited settings, benefiting populations where ECMO is scarce or nonexistent.
Considerations for Intravascular Respiratory Assist Catheter Design
The lungs are highly efficient at exchanging gas between the atmosphere and bloodstream utilizing a large surface area to volume ratio combined with a thin respiratory membrane. The respiratory surface area of the adult lung is 50–100 m2 (approximately a quarter to a half the area of a tennis court) packed into a 4 L volume.21 The alveolar-capillary membrane thickness is ∼ 0.3 µm, placing alveolar O2 in relative close proximity to red blood cells (RBCs). Deoxygenated blood is continuously supplied to the pulmonary vasculature to ensure that a favorable O2 diffusion gradient from the alveolus to the RBCs is maintained. Hemoglobin’s high binding affinity for O2 at low (venous) O2 concentrations further enhances this concentration gradient, accelerating O2 diffusion.
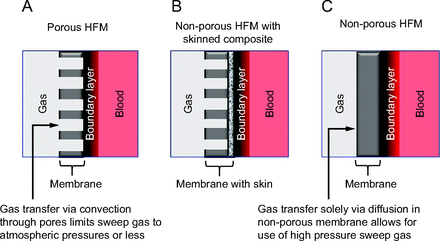
Reproducing the efficacy of the lung remains a daunting task, particularly when trying to accomplish significant gas exchange within the confines of the intravascular space. However, intravascular gas exchange can be optimized by focusing on the fundamentals of gas transport (Fig. 2). Incorporating the largest diffusing surface area possible must be balanced by the inherent size constraints of the blood vessel in which the catheter is placed so as not to significantly reduce blood flow.9,22 Depending on the ultimate efficiency of the gas-exchanging membrane, and therefore the number and density of HFMs required, an integrated microaxial pump within the catheter may be necessary to overcome the resistance to blood flow within the device. Catheter material used for gas exchange should have high O2 and CO2 diffusion coefficients, and membrane thickness should be minimized.23 The diffusion gradient can be maximized by using 100% O2 sweep gas flowing within the HFMs and can further be increased by using hyperbaric pressures.24 Whereas porous materials (Fig. 2A) inherently have high gas permeability, the risk of bubble formation via convective gas transfer limits the sweep gas to atmospheric pressures. Using a porous material with a thin-skinned composite (Fig. 2B) enhances gas permeability while also limiting plasma leakage or “wetting” into the pores that can diminish O2 transfer rate and increase thrombogenicity.23 Nonporous material (Fig. 2C) allows for high-pressure sweep gas to be used safely because gas transfer into the blood occurs solely via diffusion, thereby reducing the risk for convective bubble formation. In all cases, gas exchange will be greatest when the catheter is in contact with blood that has the lowest O2 concentration, that is, the venous system. Therefore, such a device should be deployed in a large central vein, ideally spanning the right atrium, to be exposed to the maximum amount of venous return. Finally, incorporating mechanisms to enhance mixing to disrupt laminar blood flow around the catheter, thereby reducing boundary layers, will further increase gas transfer.25-29
Gas transport across gas permeable A: porous hollow fiber membrane, B: non–porous hollow fiber membranes with skinned composite, and C: non–porous hollow fiber membrane. HFM = hollow fiber membrane.
Safety considerations include risks associated with any intravascular catheter, such as thrombus formation, thromboembolism, infection, bleeding, and tissue injury during device placement/exchange. An intravascular respiratory assist catheter will likely have an increased risk of thrombus formation when compared to a standard central venous catheter as it is assumed it would have a larger effective diameter and surface area. Catheter design, therefore, should minimize resistance to blood flow to mitigate this risk. Other methods to improve hemocompatibility should be considered, such as application of heparin-bound surface coatings.30 Another inherent risk of respiratory assist catheters include gas embolism. Such emboli could occur with catastrophic device failure, small bubble formation due to a highly efficient device that results in localized O2 supersaturation of the blood, or if a porous gas-exchanging HFM was inadvertently placed under pressure. Although the effects of pure O2 emboli are not well understood, every effort should be made to design a device that minimizes the risk of their formation given the known associated risks of air emboli.31 As it may be difficult to assess the need for and to quickly change out an intravascular gas-exchanging catheter (as compared to a circuit change in ECMO), monitoring systems designed to detect subtle fiber malfunction such as decreased efficiency due to thrombus formation, or sudden pressure loss due to gas leak, should be developed in tandem with the catheter.
Gas Exchange Requirements for a Respiratory Assist Catheter
The benefits of an intravascular gas exchange catheter exist on a spectrum dependent on the efficiency of gas exchange coupled with its risk profile. We will focus this discussion on O2 delivery to illustrate the gas exchange requirements for a respiratory assist catheter used to treat hypoxic respiratory failure. Using the pulmonary shunt fraction equation Qs/QT = (CcO2 – CaO2)/( CcO2 – CvO2) we estimated the resultant increase in arterial oxyhemoglobin saturation in varying degrees of lung dysfunction (as modeled by shunt fraction) with the addition of intravascular O2 to the venous system (Fig. 3). For simplification, we assumed a normal adult cardiac output (5 L/min), hemoglobin (12 g/dL), and arterial-venous extraction of 25%. In an intravascular venous respiratory assist catheter, the net result of O2 added by the catheter is an increase in the mixed venous O2 saturation. For example, a catheter providing 50 mL O2 min−1 would increase the arterial O2 saturation to 88%, the goal in ARDS Network studies, in a patient breathing room air (FIO2 0.21, Fig. 3A) with lung disease represented by a shunt fraction of 0.4.13 When combined with supplemental O2 (FIO2 0.6, Fig. 3B), a catheter providing approximately 110 mL O2 min−1 would raise the arterial O2 saturation to about 88% in a patient with more severe lung disease (shunt fraction of 0.6). In patients with lung disease resulting in shunt fractions > 0.6, catheters would need to deliver > 150 mL O2 min−1 or be used in conjunction with positive-pressure mechanical ventilation, which would recruit lung and improve shunting, to reach target oxyhemoglobin saturation of > 88%. Using these examples, we estimate a minimum of approximately 50 mL O2 min−1 is required to be delivered intravascularly for a clinically impactful device. Delivering intravascular O2 in this amount should allow for decreased reliance on other modes of respiratory support as previously described. This is in line with the previous findings of Conrad et al who showed the clinical benefits of the IVOX device that delivered 40–70 mL O2 min−1.10
Arterial oxyhemoglobin saturation as a function of theoretical intravascular catheter oxygen delivery. Shunt fractions are used to represent varying degrees of lung disease. A: Patient breathing air (FIO2 0.21). B: Patient receiving supplemental oxygen with FIO2 0.6.
Previous Respiratory Assist Catheters
In the following sections, the characteristics of previous attempts at developing respiratory assist catheters are presented in approximate chronological order. Lessons learned from these previous attempts at intravascular gas exchange are highlighted and discussed. A summary of the main characteristics of each device is presented in Table 1.
Summary of Prior and Current Intravascular Gas Exchange Research and Devices
IVOX Catheter
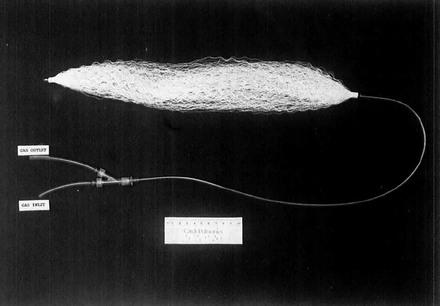
The only intravascular gas exchange device tested in human clinical trials has been the IVOX device developed by Mortenson and colleagues beginning in the 1980s at Cardiopulmonics (Salt Lake City, Utah).42 The IVOX device was an oxygenator composed of hundreds of HFMs (Fig. 4). It was designed for use within the vena cava, where venous blood could flow passively around the external surfaces of the fibers. Sub-atmospheric pressure O2 was pulled via vacuum through the lumens of the hollow fibers.43 The fiber membranes were composed of porous polypropylene covered with an ultrathin, selectively gas permeable, siloxane membrane that allowed gas transfer but no water nor plasma exchange. These fibers were crimped to minimize fiber clumping and to disrupt blood flow and boundary layer formation to enhance efficiency. The device was surgically inserted via venotomy into either the right internal jugular or femoral vein.43 Various sizes were tested clinically ranging from 38–48 Fr (1.26–1.6 cm) in diameter in the furled insertional configuration and 30–40 cm in length.32 These devices had a surface area ranging from 0.2–0.5 m2.43
Photograph of preliminary IVOX device. The crimped hollow fibers were furled into a compact bundle for insertion then unfurled within the vena cava lumen. From Reference 43, with permission.
The IVOX device was tested in a clinical trial from 1990–1993 in 160 subjects with acute hypoxic and/or hypercapnic respiratory failure receiving positive-pressure ventilation.10 Patients were selected for testing if they remained hypoxic (PaO2 < 60 mm Hg) despite FIO2 > 0.5 with one of the following ventilator requirements: PEEP ≥ 10 cm H2O, peak inspiratory pressure ≥ 45 cm H2O, or mean arterial pressure ≥ 30 cm H2O; or if they were hypercapnic (PaCO2 > 40 mm Hg) with minute ventilation > 150 mL min kg. On average, the device was capable of transferring 40–70 mL min of O2 and CO2 into or out of the circulating venous blood (O2 flux ranging from 142–219 mL O2 min−1 m−2), which is approximately 20–30% of a patient’s baseline metabolic requirement.10 At these rates of gas transfer, mechanical ventilation was reduced by 25% or more of pre-IVOX level of intensity in approximately 50% of clinical trial patients. Clinically recognized IVOX-related complications such as bleeding during insertion or explantation, venous thrombosis, and vascular obstruction were reported in 24.5% of the clinical trial subjects. The effect of device insertion on hemodynamics was appreciable with a 12.5% decrease in average cardiac index noted upon insertion of device (4.0–3.5 L min−1 m−2). 17.7% of IVOX devices were reported as having significant mechanical and/or performance malfunction problems (eg, broken fibers, failed potting, unfurling malfunction).10
Mortenson and colleagues42 were the first and only to show that clinically important gas exchange is possible with an intravascular device in humans. Unfortunately, there was no control arm in the clinical trial to determine the effect of IVOX on survival. Ultimately, the risks associated with the IVOX catheter seemed too high to justify use when balanced with the benefit of gas exchange provided. The IVOX device did not gain FDA approval. Cardiopulmonics, Inc eventually discontinued the attempt for FDA approval and halted further device development. The IVOX experience highlights the need to increase the gas exchange efficiency of intravascular devices as well as limit their size.
Hattler Catheters
The Hattler Catheter, developed by Hattler and Federspiel beginning in the late 1980s at the McGowan Institute for Regenerative Medicine at the University of Pittsburgh, was a total respiratory assist catheter composed of a bundle of HFMs with sub-atmospheric O2 flowing within.33 This group developed several prototype iterations that incorporated various methods to enhance gas exchange efficiency. They accomplished some of the highest gas fluxes reported in the literature.
One of their early prototypes consisted of 600 polypropylene HFMs (240 and 300 µm inner and outer diameter, respectively) with a diffusing surface area of 0.17 m2 bundled around a pulsating balloon capable of inflating and deflating at 300 beats/min.9 This pulsating balloon pumped blood through and around the bundle of HFMs at greater velocities than would otherwise be achieved with passive flow in the vena cava. It was the first device to incorporate active mixing into a respiratory assist catheter. The pumping action of the balloon increased the gas transfer efficiency of the device by about 200–300% depending on vessel sizes, blood flows, and pulsation rates when tested ex vivo and in vitro.9,44 However, when these catheters were tested in vivo in large-animal models, they found a more modest increase in gas exchange of only 30–40% with balloon pulsation.45-47 In water this device achieved an O2 flux of 140 mL O2 min−1 m−2, corresponding to 24 mL O2 min−1 total O2 delivery. They were unable to accurately measure O2 delivery in vivo. The CO2 transfer rate in vivo was 305 mL min−1 m−2 with a total gas exchange of 52 mL CO2 min−1. They found their largest balloon (40 mL during inflation) resulted in a significant reduction in cardiac output when implanted in 90–100 kg calves. This device never made it to clinical trials.9
Another iteration of the Hattler Catheter developed in the 2000s, the percutaneous respiratory assist catheter, consisted of 525 HFMs potted within a manifold capable of rotating at 12,000 RPM.28 Rotation was intended to induce mixing and convection of oxygenated blood away from the fiber bundle into the bulk and increase the relative velocity of blood flow past the fiber surfaces, thereby enhancing gas exchange efficiency. Using this rotational design, they were able to construct a device with a smaller insertional diameter and total diffusing surface area of 8.3 mm and 0.1 m2, respectively, when compared to their pulsating balloon device.28 The efficiency of O2 exchange, or flux, increased to 370 mL min−1 m−2 in blood for a total O2 delivery of 37 mL O2 min−1. Preliminary hemolysis results associated with operation of the percutaneous respiratory assist catheter device were similar to that of a commercially available intra-aortic balloon pump used as a control, although they noted high variability of these results.28 Given the concern for vascular endothelial damage due to the rotating fiber bundle within the vena cava, Hattler and Federspiel engineered a third device composed of an impeller within the hollow fiber bundle.34 This impeller percutaneous respiratory assist catheter was a 30-cm long 250-HFM bundle (total diffusing surface area of 0.07 m2) wrapped around a wire coil/cage containing mounted impellers of various geometries (Fig. 5). The impeller was capable of rotating up to 20,000 RPM. This design demonstrated CO2 exchange rate of 523 mL min−1 m−2 in vivo (for a total of 36 mL CO2 min−1), an increase of 70% when compared to their original balloon pulsating catheter. Oxygenation data were not reported for the device as the team began shifting their focus to CO2 removal (in part due to the continued imprecision for in vivo O2 delivery measurements). The impeller percutaneous respiratory assist catheter did not progress beyond large-animal studies.
The impellar percutaneous respiratory assist catheter consisting of 250 hollow fiber membranes potted within a manifold capable of rotating at 20,000 RPM. This device never progressed past large animal studies. From Reference 34, with permission.
These devices developed by Hattler and Federspiel represented an important advance in respiratory assist catheter technology. They were able to increase O2 flux by about 70% compared to the IVOX catheter with their ability to incorporate methods of mechanical mixing. Even with these methods of mechanical mixing, they were able to engineer and test prototypes with much smaller diameters than the IVOX catheter (∼ 8 vs 12–16 mm, respectively). However, due to the overall low total O2 transfer of these prototypes, Hattler and Federspiel focused efforts on CO2 removal as well as development of total artificial extracorporeal lungs.
Dynamic Intravascular Lung Assist Device
At the same time that Hattler and Federspiel were developing the balloon pulsating respiratory assist catheter, a group from Northwestern University developed the dynamic intravascular lung assist device. This device was the first to use movement of the gas diffusing surface of the catheter as a means to enhance the gas exchange efficiency. It used rotation and oscillation of the HFMs to increase gas transfer and reduce resistance to blood flow across the device.25,35 This device preceded that of the rotating catheter developed by the Hattler group by about a decade. Similar to the other gas exchange catheters, the dynamic intravascular lung assist device was intended to be placed within the vena cava. The device was composed of thousands of microporous polypropylene fibers (one iteration had 3,800 with a total diffusing surface area of 0.29 m2) potted in a manifold forming a sheet that was twisted around a supporting shaft, forming a screw-like configuration.35 The rotation of the fibers enhanced gas transfer and also served to pump blood forward, thereby decreasing its resistance to flow. Their most efficient device could exchange 208 mL O2 min−1 m−2, or a total of 60 mL O2 min−1, when tested in vitro using bovine blood.35 They showed that oscillatory motion increased gas exchange when compared to continuous rotation of the fiber bundle; however, the pumping effect of the screw-like configuration was lost with oscillation. Despite this device having active mixing, it did not result in an increase in O2 flux when compared to the stationary IVOX catheter (208 mL O2 min−1 m−2 compared to 142–219 mL O2 min−1 m−2, respectively). However, it did represent a significant advance in that there was no pressure drop across the screw-like device, even with blood flow at 5.9 L min−1.35 This achievement is particularly important as respiratory assist catheters are intended to function in the vena cava where passive blood flow under low pressure is easily disrupted. Ultimately the Northwestern University group’s efforts seemed to shift to the development of total artificial lungs due to the concern for vascular damage considering the rapid rotation and oscillation required with their approach.
Intracorporeal Oxygenator With Woven Tubes
Beginning in the 1990s, a group from Keio University in Japan, in collaboration with others at Penn State and Brown University, developed a prototype intravascular oxygenator device using large woven hollow tubes as a means to induce convective mixing among blood flow streams. This device consisted of 8 large 1.8 mm diameter microporous polytetrafluoroethylene tubes (total diffusing surface area of 0.05 m2) with an approximate insertional diameter of 1 cm. Interestingly, they tested this device both with blood flowing external to the tubes (with O2 flowing internally) and vice versa. Despite the finding that the woven design improved gas exchange compared to straight tubes, this design only achieved an O2 transfer efficiency of 40 mL O2 min−1 m−2 (total O2 delivery not reported) at low blood flows of 25 mL min−1.36 Increasing blood flow beyond this slow rate did little to increase the O2 exchange efficiency due to the high ratio of blood priming volume to gas-exchanging surface area within the test conduit. This high ratio highlights the importance of minimizing “shunting” of blood past respiratory assist catheters to ensure maximal contact of blood with gas diffusing surface area. It appears that development efforts halted after this initial reporting.
Penn State Intravascular Lung
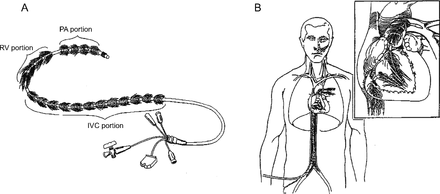
The Penn State Intravascular Lung (PENSIL) was developed in the mid-1990s and was designed to be deployed into the vena cava, right atrium, right ventricle, and the pulmonary artery to maximize exposure to the entire venous return (Fig. 6).37 Placing the catheter within the pulsatile flow of the right ventricle and pulmonary artery was proposed to enhance mixing. The 100–150-cm-long catheter was composed of thousands of blind-ended microporous polypropylene HFMs attached radially to a catheter through which O2 flowed. It was hypothesized that fibers only attached to a manifold on one end could reduce the insertional size of the device and reduce areas of tight packing, which theoretically could diminish thrombus formation. O2 was cycled in and out of the blind-ended fibers, or pressure held continuously in some, whereas a vacuum was placed in others to help with CO2 clearance. A device with 0.5 m2 diffusing surface area tested in vitro was only capable of transferring 15–20 mL O2 min−1 (or a flux of 35 mL O2 min−1 m−2).37 The PENSIL catheter is the only catheter in the literature to use blind-ended HFMs rather than hollow fibers with a gas inlet and outlet. Despite efforts at pressure cycling, the buildup of desorbed gas (CO2 and water vapor) and stagnation of gas within the blind-ended fibers reduced the driving forces for gas exchange in this device.37 This work demonstrated the importance of maintaining adequate “sweep” flow through the HFMs so as not to diminish the O2 concentration from back diffusion of water vapor or CO2. Testing did not progress beyond benchtop in vitro studies, and no further attempts at intravascular gas exchange utilized blind-ended fibers.
The Penn State Intravascular Lung (A) was designed to be deployed in the vena cava, right atrium, right ventricle, and the pulmonary artery to maximize exposure to the entire venous return. (B) It is the only device reported in the literature that used blind-ended hollow fiber membranes. From Reference 37, with permission. PA = pulmonary artery. RV = right ventricle. IVC = inferior vena cava.
Intravascular Pumping Oxygenator
The intravascular pumping oxygenator developed at Hiroshima University by Sueda and colleagues in the late 1990s was a respiratory assist catheter combined with a balloon pumping action for circulatory support.26 This unique device was composed of 40 nonporous silicone hollow fiber balloons that expanded when gas was delivered into them. In contrast to other previous respiratory assist catheters, this device was intended to be deployed into the aorta to provide both gas exchange and circulatory support. Each expanding fiber was 1 mm in diameter (when deflated) with 50 µm wall thickness. This device consisted of fewer and larger fibers than most attempts at intravascular gas exchange in order to facilitate its pumping action. An artificial “heart” driver delivered O2 into the fibers during the balloon inflation phase with a maximum pressure of 120 mm Hg while a vacuum pulled gas out of them during the deflation phase.26 It is the first device reported in the literature that used a nonporous material to prevent gas emboli, which allowed positive-pressure O2 to inflate the silicone balloons during “systole.” It appears they designed this device to operate to a maximum of 120 mm Hg of O2 pressure during balloon inflation to prevent catastrophic rupture of the membrane and to keep pressures generally equivalent to that of normal adult systolic blood pressures, which, in case of a membrane rupture, would limit the convective gradient for gas flow. The device had a total gas diffusion surface area of 0.033 m2 during the inflated phase and was able to deliver 6.3 mL O2 min−1 for a total flux of 191 mL O2 min−1 m−2 in an experimental ex vivo model.26 It was able to transfer 4.2 mL CO2 min−1 at maximum pumping blood flows of 250 mL min−1. The gas exchange provided was too low to be clinically important, and it is unclear if development efforts halted after these initial results were published.
Polyamide Hollow Fiber Intravascular Oxygenator Device
In the late 1990s a group from Tokyo Metropolitan University developed a novel asymmetric fluorinated polyimide HFM intended for intravascular gas exchange.39 This work appears to be the first reported in the literature where a polymer was developed specifically for the application in an intravascular gas exchange device. This HFM had a defect-free outer skin as thin as 10 nm to prevent plasma wetting with a microporous inner surface to enhance gas transfer (Fig. 7). Mathematical modeling showed this material to be superior to the microporous polypropylene used by others with possible O2 transfer rates 2–3 times higher.38 This group reported they had implanted these polyimide hollow fibers in a large-animal model without evidence of thrombus formation or fibrin deposition, though the gas exchange data were not reported.39 Over time it appears their efforts focused on using this material for extracorporeal membrane oxygenators.
Scanning electron microscope photograph of a cross section of the novel asymmetric fluorinated polyamide hollow fiber membrane developed for intravascular gas exchange. This membrane had a 10-nm defect-free outer skin with a microporous inner surface. From Reference 38, with permission.
Intravascular Lung Assist Device
In the early 2000s a group from Chonbuk National University in South Korea began work on intravascular oxygenation that culminated in prototypes utilizing high-frequency vibration to enhance gas exchange.40 They were the first and only reported in the literature to use high-frequency vibration in this application. Intravascular lung assist device prototypes consisted of microporous polypropylene HFMs wrapped around a multilayer bender piezoelectric actuator. Vibration was intended to decrease the thickness of the blood boundary layer, which by this time was well known to be a key area of resistance to mass transfer in gas exchange devices. They developed proof-of-concept extracorporeal prototypes with bundles ranging from 100–675 HFMs placed in a 30 mm diameter conduit. Maximum O2 transfer efficiency occurred when vibrating the bundle of HFMs at 7 Hz, with a gas exchange improvement of 52% when compared to static fibers. O2 delivery of 57 mL O2 min−1 was obtained in blood with the largest prototype (surface area of device not reported; therefore, O2 flux also unknown). Importantly, they showed that whereas hemolysis did occur with their vibrating HFM bundles it was approximately within the range of commercially available centrifugal blood pumps used in extracorporeal mechanical circulatory support (normalized index of hemolysis of 36–95 compared to 34–61, respectively).48 Work has continued on the development of intravascular lung assist devices, though the focus has shifted to improving gas delivery by development of a microencapsulated hemoglobin hemosome that has a high affinity for O2 binding, intended for simultaneous intravenous infusion during device operation.49
Highly Integrated Intravascular Membrane Oxygenator
The highly integrated intravascular membrane oxygenator device was developed by a group at the Helmholtz Institute in Germany in the early 2000s (Fig. 8).41 This device was the first to integrate a pump within a catheter to overcome the pressure drop and resistance to flow produced by tightly packed HFMs. This device combined a microaxial blood pump upstream of a bundle of disc-shaped polyolefin HFMs. They constructed 4 prototype bundles containing 480–624 hollow fibers to investigate the effects of fiber density and porosity on gas exchange, though notably none of these proof-of-concept devices included the microaxial pump.27 At 3 L min−1 of blood flow, the device achieved O2 transfer efficiency (flux) of 450 mL O2 min−1 m−2, though only a total gas delivery of 6 mL O2 min−1 due to the small surface area (not reported). At the time, this represented the highest reported flux in the literature. CO2 clearance was not investigated. Despite the impressive O2 exchange efficiency, it appears that further development of HIMOX halted due to the low overall total O2 delivery.
3D representation of the highly integrated membrane oxygenator device. Note the fiber bundles (A) are twisted (B) to deploy them into the final cross-flow configuration (C) upon placement in the vena cava. From Reference 41, with permission.
Current Efforts: The IntraVascular Membrane Oxygenator
The IntraVascular Membrane Oxygenator catheter currently in development by our team is the most recent published effort at developing a respiratory assist catheter.24 In contrast to previous attempts at intravascular gas exchange, this novel approach relies specifically on hyperbaric O2 to generate a large driving concentration gradient for diffusion across nonporous amorphous fluoropolymer HFMs rather than relying solely on a large surface area. This device is only intended to deliver O2, the primary deficit in most forms of acute lung injury. This approach may allow for smaller HFM surface area for a more compact device amenable to intravascular use.
Our HFM system has been designed to operate at up to 2 bar (30 PSI) to date, approximately 12 times higher than the pressure used by the intravascular pumping oxygenator developed by Sueda and colleagues. The current HFM material used is Teflon AF2400 with an outer diameter of 406 µm and wall thickness of 89 µm (Biogeneral, San Diego, California). Modeling predicts full-scale prototypes will utilize 100–200 fibers, 25–30 cm in length. Teflon AF2400 was chosen for high O2 gas permeability of 9.9 × 10−8 cm2 s cm Hg and sufficiently high burst strength (theoretically > 130 bar), allowing operation at high gas pressures.50 In vitro testing in water demonstrates O2 flux of approximately 550 mL min−1 m−2 when a single fiber was isolated in a well-mixed system and 350 mL min−1 m−2 when a bundle of fibers are tested in a flow-through system similar to intravascular conditions. The O2 flux of our approach may be even greater in blood as suggested by the work converting water oxygenation data to blood by Vaslef and associates (also confirmed by Hattler’s group) who reported up to a 2-times increase.9,51 It is important to note, however, that small O2 bubbles have been observed to form alongside the static HFMs when operating in water at higher pressures (1.9 bar) and/or when the bulk water–dissolved O2 concentrations were high. Consequently, our team is working to incorporate unique methods of mixing with the goal to enhance O2 flux and eliminate bubble formation. Overall, these feasibility studies demonstrate that a hyperbaric approach to intravascular membrane oxygenation has the potential to deliver a clinically important quantity of O2 in a device that is more compact than previous attempts at intravascular gas exchange.
Summary
Physicians, physiologists, and biomedical engineers have collaborated over the past 40 years to develop devices intended for intravascular gas exchange through various technical approaches. Most had limited success, with only one device, the IVOX catheter, tested in human clinical trials before development was halted without FDA approval. Each attempt at intravascular gas exchange provided insight on how to optimize conditions affecting gas transport, thereby allowing subsequent-generation devices to overcome these technical challenges. Respiratory assist catheters that are highly efficient and provide a physiologically relevant amount of gas exchange could play an important role in limiting/avoiding toxic mechanical ventilator support and preventing the need for more invasive support such as VV-ECMO. If successfully developed, their use would represent a major conceptual shift in the way physicians currently treat respiratory failure as they could provide patients with a minimally invasive alternative treatment capable of providing gas exchange independent of diseased lungs.
Footnotes
- Correspondence: Tobias L Straube MD, Duke University Medical Center, 2301 Erwin Rd, Durham, NC 27710. E-mail: tobias.straube{at}duke.edu
Dr Straube is supported by NICHD of the National Institutes of Health under award number T32HD094671.
Drs Straube, Klitzman, Cheifetz, and Vesel have disclosed that they are listed as inventors on US patent application 15/950,517, Intravascular Membrane Oxygenator Catheter Systems and Methods. Drs Straube, Farling, Deshusses, Klitzman, Cheifetz, and Vesel have disclosed that they are listed as inventors on patent application 63/170,045, Intravascular Membrane Oxygenator Catheter.
- Copyright © 2022 by Daedalus Enterprises