Abstract
BACKGROUND: Children with asthma exacerbations requiring pediatric ICU (PICU) admission, known as critical asthma (CA), are prescribed a variety of therapeutic interventions including heliox. Delivered invasively and noninvasively, heliox is employed to enhance deposition of aerosolized medications, improve obstructive pulmonary pathophysiology, and avoid complications associated with invasive mechanical ventilation. We used the Virtual Pediatric Systems database to update estimates of heliox prescription and explore for relationships between heliox and mechanical ventilation frequency and duration.
METHODS: We performed a retrospective cohort study using data from 97 PICUs among children 3–17 y of age admitted for CA from 2013–2019. The primary outcome was heliox prescribing rates and trends. Subgroup analyses assessed mechanical ventilation rates and duration by heliox exposure.
RESULTS: Of 43,238 subjects studied, 1,070 (2.5%) were prescribed heliox. Mean heliox prescribing rates fell from 4.11% in 2013 to 2.37% in 2019. Heliox use was greater from centers in the South (2.6%) and Midwest (3.3%) as compared to the West (1.6%) and Northeast United States (1.6%, P < .001). In the subgroup assessing mechanical ventilation frequency, mechanical ventilation rates were 273/39,739 (0.7%) and greater for those provided heliox (1.9% vs 0.7%, P < .001). In the subgroup assessing mechanical ventilation duration, no differences in median mechanical ventilation duration were observed (4.94 [interquartile range [IQR] 3.04–6.36] vs 4.63 [IQR 3.11–7.30] d; P = .35) for those with and without heliox. In exploratory adjusted models, noninvasive heliox was not associated with mechanical ventilation. Mortality was rare (206/43,238 [0.47%]) and predominantly among subjects intubated prehospitalization (188/206 [91.3%]).
CONCLUSIONS: Heliox as adjunctive therapy for children with CA is uncommon (2.5%) and not associated with mechanical ventilation or decreased mechanical ventilation duration in adjusted models. Updated estimates provided herein inform prospective controlled trial development to better define the role of heliox for CA.
- heliox
- critical asthma
- status asthmaticus
- pediatric ICU
- mechanical ventilation
- mortality
- invasive ventilation
Introduction
Nearly 5.5 million children in the United States have chronic asthma, and exacerbations account for over 75,000 hospitalizations and 626,000 emergency department visits annually (https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm, Accessed July 1, 2021).1,2 Exacerbations are characterized by severe bronchospasm, mucos production, and inflammatory dysregulation acutely treated with systemic corticosteroids and nebulized bronchodilators. With critical asthma (CA), worsening respiratory acidosis and impaired cardiopulmonary interactions3-5 call for advanced monitoring and adjunctive interventions in the pediatric ICU (PICU) setting. Invasive mechanical ventilation is generally avoided to limit complications and acquired comorbidities after transitioning from spontaneous ventilation to positive-pressure ventilation.6,7
In the PICU, adjunctive pharmacologic and respiratory therapies are applied for CA including heliox, a mixture of helium and oxygen.8,9 The proposed mechanism of action for heliox includes a reduction in Reynold number, yielding laminar air flow in narrowed airways10-12 and enhancing the deposition of aerosolized medications.13 Yet, limited data are available to suggest heliox, as with nearly all other adjunctive therapies, improves clinical outcomes in pediatric CA such as pulmonary function or reduces hospital length of stay (LOS).14-20 The absence of convincing evidence to support heliox for CA is multifactorial and related to heterogenous study populations (eg, children and adults), single-center sampling, inconsistent heliox mixtures used, and varying institutional practices. We anticipated the proposed mechanism for heliox has potential to reduce the frequency and duration of mechanical ventilation by improving air flow and β-agonist distribution to narrowed airways as seen in asthma pathophysiology. Estimated mechanical ventilation rates for CA are 1–7% and largely based upon single-center experiences.5,18,21 To date, there are no data to infer heliox applied noninvasively reduces mechanical ventilation rates or ventilation duration if applied after endotracheal intubation. Prior to controlled interventional trials to address these knowledge gaps, updated estimations for heliox use, mechanical ventilation, and ventilation duration in cases of pediatric CA are needed.
Therefore, we used a multi-center database, the Virtual Pediatric Systems (VPS) registry, to describe heliox prescribing rates chronologically, cumulative CA admission volume by site, and by United States region for children admitted to the PICU for CA. We hypothesized that variation in heliox prescribing exists chronologically, geographically, and by cumulative center CA admission volume. In an exploratory fashion, we also hypothesized subjects exposed to heliox prior to mechanical ventilation will have a reduced frequency of intubation, and those endotracheally intubated for CA with invasive heliox have a reduced ventilation duration.
QUICK LOOK
Current Knowledge
Heliox is prescribed to children admitted for critical asthma (CA) in the pediatric ICU as a rescue therapy to avoid mechanical ventilation, improve deposition of aerosolized medications, and enhance laminar flow in obstructed conducting airways. Yet, there are inadequate descriptive and comparative data to support the routine use of heliox as adjunctive therapy for children hospitalized for CA heterogeneity.
What This Paper Contributes to Our Knowledge
In this multi-center, retrospective study data from 43,238 encounters from 97 children’s hospitals, we noted a decline in mean heliox prescribing from 4.11% in 2013 to 2.37% in 2019 for CA and mean invasive mechanical ventilation rate of 4.77%. Noninvasive heliox was associated with subsequent invasive ventilation but no differences in duration in mechanical ventilation when applied after endotracheal intubation.
Methods
Data Source
We conducted a multi-center, retrospective cohort study using in-patient encounter data from January 1, 2013–December 31, 2019, from the VPS registry (Virtual Pediatric Systems, Los Angeles, California). This registry represents data prospectively collected through an international, voluntary collaborative including deidentified data from more than 200 PICUs with over 1.5 million individual encounters over the past 20 years. This study was reviewed and approved by the Johns Hopkins All Children’s Hospital Institutional Review Board (IRB no. 00220354).
Study Participants and Cohorts
Inclusion criteria were subjects 3–17 y of age admitted to the PICU in the United States with principal diagnoses corresponding with CA using System Tracking for Audit and Reimbursement and International Classification of Diseases, 9th/10th revisions, Clinical Modification diagnostic codes. Exclusion criteria included the presence of congenital heart disease, interstitial lung disease, acute chest syndrome, bronchopulmonary dysplasia, cystic fibrosis, pulmonary hypertension, tracheostomy dependence, laryngotracheobronchitis, bronchiolitis, and neuromuscular disease. Children < 3 y of age were excluded to avoid misclassification with diagnoses such as laryngotracheobronchitis or bronchiolitis. A comprehensive list of coding corresponding to inclusion and exclusion criteria is listed in Supplemental Table 1 (see related supplementary materials at http://www.rc.rcjournal.com). To improve sample homogeneity and generalizability, the distribution of sites was separated into hospital center tertiles (ie, small, medium, and large centers) by cumulative CA admission volumes throughout the study period. Data from small centers were excluded a priori to account for bias from low-volume centers that may be less familiar with heliox and CA management.
The study sample was assessed by cohorts defined by presence or absence of heliox exposure. Heliox and mechanical ventilation are manually reported into VPS including start and stop dates and times. A heliox–mechanical ventilation frequency subgroup was used to assess the impact of noninvasive heliox on observed rates of mechanical ventilation by applying additional exclusion criteria including mechanical ventilation prior to admission (to account for intubation in the field or at an outlying facility), mechanical ventilation within the first 6 h of admission (suggesting insufficient exposure to heliox), mechanical ventilation prior to heliox (to infer chronologic plausibility), and for encounters with absent Pediatric Risk of Mortality III (PRISM III) data. Next, a heliox–mechanical ventilation duration subgroup was created to evaluate the impact of heliox applied concurrent to mechanical ventilation on ventilation duration by excluding subjects where heliox was not administered during mechanical ventilation, in cases where heliox exposure was < 6 h, when no PRISM III data were reported, and for subjects intubated < 2 d (to account for subjects intubated to facilitate safe transfer). Unlike the heliox–mechanical ventilation frequency subgroup, the heliox–mechanical ventilation duration subgroup included subjects intubated within 6 h of admission or prior to hospitalization.
Study Definitions and Outcomes
The primary study outcome was heliox exposure assessed chronologically by study year, participating center, cumulative center CA admission volume (“medium” vs “large” centers), and United States Census geographic region (Midwest, Northeast, South, and West). The secondary exploratory outcome was mechanical ventilation rate evaluated in the heliox–mechanical ventilation frequency subgroup as outlined above. The tertiary exploratory outcome was mechanical ventilation duration from the heliox–mechanical ventilation duration subgroup as outlined above. Use of the terminology “mechanical ventilation” refers to endotracheally intubated subjects. Descriptive data were age, gender, race, PICU LOS, hospital LOS, index mortality, Pediatric Index of Mortality 2 and 3 risk of mortality (PIM-2/3 ROM) scores, PRISM III probability of mortality (POM), intubation timing in relation to PICU admission timing, extracorporeal life support (ECLS) rate, tracheostomy rate during admission, pneumothorax rate, and noninvasive respiratory support (NIRS) rate. Modalities of NIRS included continuous positive airway pressure; bi-level positive airway pressure; and heated, humidified high-flow nasal cannula.
Statistical Analyses
Descriptive statistics are reported as proportions with percentages, means ± SD, or medians (interquartile range [IQR]) depending on data distribution and type. For categorical data, chi-square and Fisher exact tests were used to compare proportions. Lilliefors (Kolmogorov-Smirnov) tests were used to assess continuous data distribution and normality. Students t and Mann-Whitney (Wilcoxon rank sum) tests were employed to compare parametric and nonparametric continuous data, respectively. Chronologic trends in mean annual center heliox prescribing rates were assessed using Joinpoint regressions (Joinpoint software v4.7, National Cancer Institute, Bethesda, Maryland). To assess for potential associations between heliox and mechanical ventilation, unadjusted and adjusted logistic regression were used to calculate an odds ratio (OR) with 95% CI. To assess for potential associations between heliox exposure and mechanical ventilation duration, unadjusted and adjusted linear regression were employed. Significant independent variables from unadjusted analyses were added as covariates in adjusted models (if P < .1). Subject age, heliox exposure, and PRISM POM were added a priori to adjusted models. Statistical tests were 2-sided, and type I error was set at 0.05. Encounters were used as the unit of analysis and assumed independent. Statistical analyses were completed using R version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Study Sampling and Cohorts
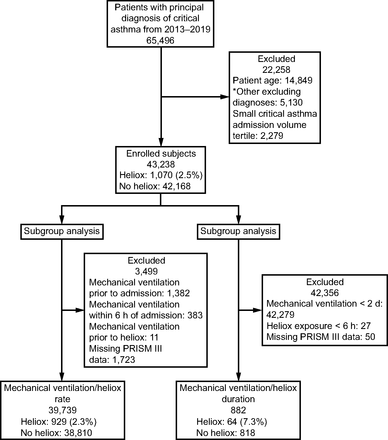
From January 1, 2013–December 31, 2019, 65,496 unique PICU encounters were identified for study. After initial study criteria were applied, 43,238 encounters from 97 centers composed the overall study sample of which 1,070 (2.5%) were prescribed heliox for a mean duration of 21.4 ± 27.2 h. For the heliox–mechanical ventilation frequency subgroup, 39,739 encounters were assessed of which 929 (2.3%) were prescribed heliox prior to mechanical ventilation for a mean duration of 19.7 ± 25.7 h. For the heliox–mechanical ventilation duration subgroup, 882 encounters were evaluated of which 64 (7.3%) received heliox concurrent to mechanical ventilation for a mean duration of 51.8 ± 63.8 h. A consort diagram depicts sampling and study cohorts (Fig. 1).
Consort diagram depicting study sampling and cohorts. PRISM III = Pediatric Index of Mortality III. *Other diagnoses: congenital heart disease, interstitial lung disease, acute chest syndrome, bronchopulmonary dysplasia, cystic fibrosis, pulmonary hypertension, tracheostomy dependance, laryngotracheobronchitis, bronchiolitis, or neuromuscular disease.
Encounter Characteristics
Demographic and severity of illness data for the overall study sample are listed in Table 1 (and for subgroups in Supplemental Table 2, see related supplementary materials at http://www.rc.rcjournal.com). Those with CA provided heliox were older (median 8.75 [IQR 5.92–12.17] vs 7.58 [IQR 5.17–11.08] y, P < .001) and more frequently Black or African American (44.7% vs 37.3%, P = .02) compared to those without heliox. Whereas statistically significant differences in available severity of illness indices (median PIM-2 ROM, PIM-3 ROM, and PRISM III POM) were in the overall sample, these differences were not noted in the subgroup analyses and may not be clinically relevant as POM suggested from these metrics was overwhelmingly low (all < 2%).
Subject Demographics, Severity of Illness Indices, Census Geographic Region, and Critical Asthma Center Admission Volume Data for the Entire Study Sample
Heliox Prescribing Patterns and Trends
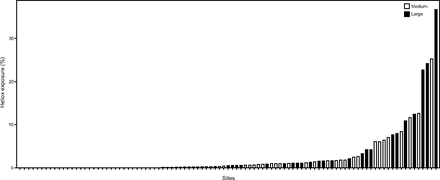
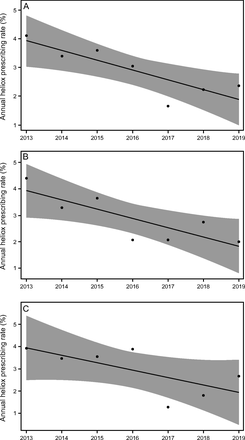
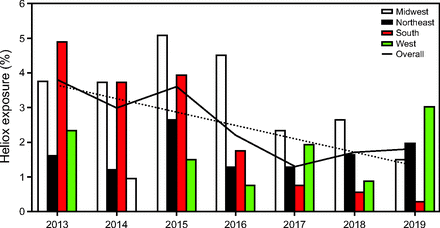
Annual heliox prescribing rates by cumulative center CA admission volume groups (medium vs large) are found in Table 2. Heliox exposure varied by individual centers with rates ranging between 0–36.78% (Fig. 2). Joinpoint regression analyses were performed to assess trends and potential annual break points throughout the study period for the overall sample and subgroups (Supplemental Table 3, see related supplementary materials at http://www.rc.rcjournal.com). These analyses revealed a simple linear model was best fit for the overall sample, with mean annual institution heliox prescribing rates falling from 4.11 ± 9.86% in 2013 to 2.37 ± 5.75% in 2019 (Figure 3A). This decline was more pronounced in medium CA volume centers where rates decreased from 4.40 ± 9.16% in 2013 to 2.00 ± 2.79% in 2019 (Figure 3B) than large CA volume centers (Figure 3C). Heliox exposure data by United States Census geographic regions are found in Figure 4. Of note, lower rates of heliox were noted among PICUs in the Northeast (1.59%) and West (1.64%) as compared to the Midwest (3.34%) and South (2.61%, P < .001) and remained significant with post hoc testing using a Bonferroni correction (type I error reset at 0.0083; data not shown).
Heliox exposure by hospital center and cumulative center critical asthma admission volume (medium vs large volume centers).
A: Mean annual heliox prescribing rates from 2013–2019 for subjects admitted to the pediatric intensive care unit for critical asthma among 97 children’s hospitals. B: Mean annual heliox prescribing rates from 2013–2019 for subjects with critical asthma admitted to 48 medium-volume pediatric intensive care units. C: Mean annual heliox prescribing rates from 2013–2019 for subjects with critical asthma admitted to 49 large-volume pediatric ICU.
Heliox exposure by year and United States Census geographic regions.
Heliox Exposure by Year and Cumulative Critical Asthma Center Admission Volume Tertile
NonInvasive Heliox Exposure and Mechanical Ventilation Frequency
During the study period, 2,064 of 43,238 (4.77%) subjects were invasive ventilated, and the median time to mechanical ventilation excluding those intubated prior to admission was not significantly different between subjects who did or did not receive heliox (5.43 [IQR 0.1–11.9] vs 4.63 [IQR 0.5–18.5] h, P = .31). After applying additional study criteria for the heliox–mechanical ventilation frequency subgroup analyses, the frequency of mechanical ventilation was 273 of 39,739 (0.69%). For subjects who received noninvasive heliox prior to intubation, 18 of 929 (1.94%) were intubated at a median of 14.95 (IQR 8.86–19.92) h after admission for a median duration of 3.57 (IQR 2.35–5.13) d. Compared to those who received heliox, subjects who did not were less frequently intubated (255 of 38,810 [0.66%], P < .001), intubated a median of 5.05 h later (20 [IQR 11.16–42.21] h after admission, P = .041), and for a similar duration (median 4.34 [IQR 2.22–7.55] d, P = .23). In unadjusted logistic models (Supplemental Table 4, see related supplementary materials at http://www.rc.rcjournal.com), variables related to subsequent mechanical ventilation included heliox exposure (OR 2.27 [95% CI 1.27–4.31], P = .007), admission to a site with greater CA admission volume (OR 0.49 [95% CI 0.37–0.66], P < .001), and lower PRISM III (OR 0.27 [95% CI 0.19–0.38], P < .001). Whereas in an adjusted logistic model, lower PRISM III (OR 0.28 [95% CI 0.20–0.40], P < .001) and admission to a large volume CA center (OR 0.48 [95% CI 0.36–0.64], P < .001) were associated with mechanical ventilation; exposure to noninvasive heliox was not associated with mechanical ventilation (OR 1.73 [95% CI 0.91–3.30], P = .10).
Invasive Heliox Exposure and Mechanical Ventilation Duration
For the heliox–mechanical ventilation duration subgroup analyses, no differences in median mechanical ventilation duration were observed between those with and without heliox exposure (4.94 [2.68–6.19] vs 4.74 [2.97–7.33] d, P = .38). In unadjusted linear models (Supplemental Table 5, see related supplementary materials at http://www.rc.rcjournal.com), heliox was not associated with mechanical ventilation duration (P = .21). In adjusted linear models, variables related to greater mechanical ventilation duration included tracheostomy placement during hospitalization (coefficient 1.19 [95% CI 0.90–1.47], P < .001) and use of ECLS (coefficient 0.49 [95% CI 0.31–0.67], P < .001).
Other Clinical Outcomes
LOS, mortality, and other clinical outcomes are listed in Supplemental Table 6 (see related supplementary materials at http://www.rc.rcjournal.com). Overall, encounters with heliox exposure experienced a longer median PICU LOS (2.18 [1.46–3.54] vs 1.33 [0.82–2.14] d, P < .001). In the invasive heliox–mechanical ventilation duration subgroup, median PICU LOS was not observably different between groups with and without heliox exposure (7.37 [5.60–9.72] vs 7.37 [5.01–11.86] d, P = .67). Mortality was rare and observed in 206 of 43,238 (0.48%). Deaths were largely accounted for by subjects intubated prior to PICU admission (188 of 206, 91.3%), and mortality was not observably lower among encounters with and without heliox exposure. Overall, those provided heliox more frequently required ECLS (0.56% vs 0.16%, P < .001) and subsequent tracheostomy (0.28% vs 0.07%, P = .048). However, for both subgroup analyses, no differences in ECLS and tracheostomy insertion rates were observed.
Discussion
In this multi-center, retrospective cohort study assessing subjects 3–17 y from 97 PICUs using the VPS registry from 2013–2019, we provide updated estimations for heliox prescribing and invasive mechanical ventilation rates for CA. Mean institution heliox prescribing rates declined from 4.11% in 2013 to 2.37% in 2019. The overall mechanical ventilation rate was 4.77% but only 0.69% after excluding subjects intubated prior to hospitalization. Mechanical ventilation was not associated with heliox exposure prior to intubation but instead related to PRISM III–POM and center CA admission volumes. These findings may represent greater severity of illness or provider practice variation. We did not detect associations between invasive heliox prescribed after intubation and mechanical ventilation duration. Mortality was 0.48%, with a majority (91%) accounted for by subjects intubated prior to hospitalization. Updated estimates for pediatric CA heliox prescribing and mechanical ventilation data provide the much-needed data to inform definitive prospective controlled trials.
Heliox, like other adjunctive CA therapies, remains a controversial and unproven treatment. Only a handful of randomized controlled trials are published using heterogenous sampling criteria, heliox concentrations, and primary end points. In 1996, Carter et al20 randomized 11 children 5–18 y of age with CA to receive either heliox 70/30 mixture or room air. They found no improvements in mean FEV1, FVC, or severity of illness scores. A similar trial by Kudukis et al22 enrolled 18 children 16 months–16 y of age with CA and found those receiving heliox 80/20 mixture demonstrated improved pulsus paradoxus, peak expiratory flow, and dyspnea scores compared to room air. In 2005, Kim et al23 randomized 30 children with CA age 2–18 y in the emergency center to receive either nebulized albuterol with concomitant heliox or oxygen. They found improved pulmonary function and a higher frequency of early discharge for children given heliox. Small randomized studies of children in the PICU and emergency department to noninvasive heliox versus oxygen found no differences in asthma severity scores, LOS, or mechanical ventilation rates.24,25 These inconsistent outcomes may represent confounding from variable heliox concentrations, sample size, study design, subject age, primary end points, existing asthma protocols, and concurrent exposure to alternative adjunctive therapies such as NIRS. Inclusion of young children may add bias as heliox is used for airway edema and flow obstruction in severe laryngotracheobronchitis. Further, young children may not reliably participate in pulmonary function testing and, therefore, cannot provide indices for objectifying therapeutic effectiveness.
In addition to randomized trials, systematic reviews from single-center experiences in adults and children have sought to assess the impact of heliox. In 2003, Rodrigo et al26 were unable to detect differences in pulmonary function related to heliox therapy in primarily adult subjects. A follow-up systematic review in 2014 including 10 publications (assessing 697 subjects from 7 adult studies and 3 from mixed pediatric and adult studies) demonstrated improved peak expiratory flows in subjects who received heliox compared to oxygen.14 We speculate these results also reflect heterogenous sampling, heliox concentration, practice adaptations throughout the study period, and varying CA management protocols.
The downtrend in heliox prescribing from 2013–2019 from our data may represent the absence of comparative effectiveness data, use of alternative adjuncts, and lack of consensus-based guidelines for standardized management. These trends are consistent with a recently published multi-center, retrospective registry-based assessed on the Pediatric Health Information System database.21 We speculate variation by centers is related to provider familiarity with heliox in lieu of competing adjunctive therapies such as terbutaline, aminophylline, or NIRS. Decreased heliox prescribing may also be attributable to heliox cost that has nearly doubled in past last decade (https://www.usgs.gov/centers/nmic/helium-statistics-and-information. Accessed December 11, 2020).27 As with our data, Hasegawa et al28 reviewed childhood asthma hospitalizations in the United States over 10 y from 2000–2009 and found the highest prescribing rates occurred in the South. Chronologic and regional variation highlights the need for prospective, controlled trials for heliox (and in comparison to alternative adjunctive therapies) for the generation of evidence to support standardized practice guidelines.
Whereas our findings were primarily descriptive, we did explore for relationships between heliox and mechanical ventilation frequency and duration. In a single-center descriptive study from Australia, Rampersad et al29 noted an overall mechanical ventilation frequency of 7% for children with CA and rates increased from 6% in 2000 to 14% in 2011. These data included children intubated at outlying referring centers. A median duration of ventilation of 1.08 d (IQR 0.58–2.19) from these reports suggests either limited disease severity or intubation was performed for transport safety. A single-center retrospective study by Carroll and colleagues30 reviewing 251 children with CA from 1997–2005 found subjects from community centers were more commonly intubated as compared to tertiary facilities (17% vs 5%). Similarly, VPS data from 2003–2006 published by Shibata et al31 noted an invasive mechanical ventilation rate of 4.6% for children, with a vast majority intubated prior to PICU admission. A recent multi-center observational study estimated mechanical ventilation rates among children with critical asthma to be 12.8% between 2010–2019.21 Differences in our reported rates likely represent alternative sampling criteria and registry-specific limitations.
As with these reports, subjects intubated prior to admission accounted for a majority of index mortalities in our sample. This emphasizes the importance of chronic asthma management, social determinants of heath, and the vital nature of preventive medicine. Although subjects exposed to heliox were more commonly intubated for CA, this may reflect greater severity of illness as indicated by greater PRISM III POM values. Yet, the performance of PRISM III as an indicator of asthma exacerbation severity during hospitalization is limited. We did not detect differences in mechanical ventilation duration for subjects provided invasive heliox and noted few complications associated with intubation or mechanical ventilation (ie, pneumothoraces, tracheostomy dependence, or use of ECLS). Association between ECLS and tracheostomy placement with longer ventilation duration was expected as these interventions inherently extended LOS.
Limitations
Erroneous data entered by participating sites may result in sampling errors, poor cohort identification, and miscalculations of study outcomes. Registry data do not include heliox concentrations (80/20, 70/30, or 60/40), and we cannot assess its potential influence as a covariate in exploratory models. Indication for heliox is not recorded in VPS, and some centers may use heliox as a standard carrier gas for continuous nebulized albuterol rather than adjunctive therapy (although its application as a carrier over air or oxygen in CA implies an intention to treat). Participating site PICU admission criteria variability (eg, age criteria, capacity to offer continuous nebulization on general pediatric wards, etc) may confound observed prescribing rates. Data in VPS are taken primarily from tertiary pediatric referral centers and may not be generalizable to community hospitals. Hypoxia, a relative contraindication for heliox, is not recorded in VPS and may account for prescribing variation. The VPS registry does not record alternative adjunctive therapies (that is, terbutaline and aminophylline, viral versus nonviral triggers) or asthma-specific severity of illness indices that would be essential to future, prospective investigation. We excluded children < 3 y of age to improve subject homogeneity, but as a result, these findings cannot be generalized to this age group.
Conclusions
In this multi-center retrospective study of subjects 3–17 y of age admitted to the PICU for CA from 97 children’s hospitals, we observed a decreasing trend of heliox prescribing from 4.11% in 2013 to 2.37% in 2019 that varied by region and CA admission volumes. Mechanical ventilation rates for pediatric CA were 4.77% and index mortality 0.69%. Most subjects were intubated prior to admission, and these cases accounted for 91% of mortalities. In adjusted models, noninvasive heliox was not associated with mechanical ventilation, and invasive heliox during mechanical ventilation was not associate with ventilation duration. These data provide contemporary estimations for heliox use, mechanical ventilation, and mortality for subjects admitted to the PICU with pediatric CA.
Acknowledgments
Gerardo Soto-Campos PhD, (Director of Analytics) and Jamie Palumbo MSc, (data scientist) from Virtual Pediatric Systems. Neil Goldenberg MD PhD and Sharon Ghazarian PhD from the Johns Hopkins All Children’s Hospital Clinical and Translational Research Training certificate program. The Johns Hopkins All Children’s Hospital Foundation.
Footnotes
- Correspondence: Anthony A Sochet MD MHSc, Department of Anesthesia and Critical Care Medicine, Johns Hopkins All Children’s Hospital, 501 6th Ave S., Suite 702A, St. Petersburg, FL, 33701; telephone: 727–767-2912. E-mail: Anthony.Sochet{at}jhmi.edu
See the Related Editorial on Page 624
Supplementary material related to this paper is available at http://rc.rcjournal.com.
The authors have disclosed no conflicts of interest.
The study was performed at Johns Hopkins All Children’s Hospital, St. Petersburg, Florida.
- Copyright © 2022 by Daedalus Enterprises