Abstract
INTRODUCTION: Awake prone positioning (PP) reduces need for intubation for patients with COVID-19 with acute respiratory failure. We investigated the hemodynamic effects of awake PP in non-ventilated subjects with COVID-19 acute respiratory failure.
METHODS: We conducted a single-center prospective cohort study. Adult hypoxemic subjects with COVID-19 not requiring invasive mechanical ventilation receiving at least one PP session were included. Hemodynamic assessment was done with transthoracic echocardiography before, during, and after a PP session.
RESULTS: Twenty-six subjects were included. We observed a significant and reversible increase in cardiac index (CI) during PP compared to supine position (SP): 3.0 ± 0.8 L/min/m2 in PP, 2.5 ± 0.6 L/min/m2 before PP (SP1), and 2.6 ± 0.5 L/min/m2 after PP (SP2, P < .001). A significant improvement in right ventricular (RV) systolic function was also evidenced during PP: The RV fractional area change was 36 ± 10% in SP1, 46 ± 10% during PP, and 35 ± 8% in SP2 (P < .001). There was no significant difference in PaO2/FIO2 and breathing frequency.
CONCLUSION: CI and RV systolic function are improved by awake PP in non-ventilated subjects with COVID-19 with acute respiratory failure.
Introduction
Prone positioning (PP) has been shown to improve oxygenation in mechanically ventilated patients.1-4 Guerin et al5 reported that PP also decreases mortality for subjects with moderate-to-severe ARDS requiring invasive mechanical ventilation. From a hemodynamic point of view, PP increases the cardiac index (CI) in this specific setting.6,7 On the one hand, the increase in CI could be related to an increase in the preload;6 on the other hand, it may also be explained by a decrease in the RV afterload, as a pulmonary vascular resistance reduction was observed.6 By contrast, Evrard et al8 did not find any improvement in CI during PP in invasively ventilated subjects with moderate-to-severe COVID-19 ARDS, whereas they all responded in terms of PaO2/FIO2. The hemodynamic consequences of PP may be a crucial issue because the reduction in mortality was partly due to a significant reduction in non-hypoxemic sudden cardiac arrest and a significant increase in cardiovascular dysfunction-free survival.5
The extrapolation of those results to awake PP in patients with COVID-19 is not self-evident. Systematic reviews on awake PP including subjects with COVID-19 and non–COVID-19 subjects8-10 showed a systematic improvement of oxygenation, despite the use of varied PP durations. Besides, this improvement was reversible once subjects returned to the supine position (SP). During the COVID-19 outbreak, awake PP has been incorporated into clinical guidelines11 and proposed in expert consensus statements.12 Awake PP was identified as a research priority by the Surviving Sepsis Research Committee.13 More recently, in a randomized controlled, multinational, open-label meta-trial, awake PP has been shown to be able to reduce the incidence of treatment failure and the need for intubation;14 those data were confirmed in a meta-analysis.15 However, the hemodynamic effects of awake PP on non-intubated subjects remain to be explored.
Therefore, we sought to investigate the hemodynamic consequences of awake PP on non-intubated subjects with COVID-19. We hypothesized that PP would improve the CI in that setting. Our main objective was to compare the CI before, during, and after PP. Secondary objectives were to describe both RV systolic function and respiratory variables before, during, and after PP.
QUICK LOOK
Current knowledge
Prone positioning (PP) mechanically ventilated patients with ARDS improves gas exchange and outcome. PP can increase cardiac index (CI) and improve right ventricular (RV) function. Prone position can also improve respiratory function and outcomes of patients with acute respiratory failure without mechanical ventilation.
What this paper contributes to our knowledge
In subjects with acute respiratory failure due to COVID 19 requiring oxygen delivery but not mechanical ventilation, we showed that there is a strong hemodynamic effect of PP. PP in awake non-ventilated subjects increases CI through an improvement of RV function partly attributable to a decrease in RV afterload.
Methods
This prospective cohort study was conducted in the ICU of the tertiary teaching Louis Pradel Hospital (Lyon, France) from October 2020–May 2021. A local review board approved the study protocol (AGORA registration number: A330); a declaration of data set creation was done to the French commission on data protection (Comission nationale de l’informatique et des libertés, registration number: 21 5330), and the study was then registered (ClinicalTrials.gov: NCT04834947). Informed consent requirement was waived by the ethics committee. We followed the Standards of Reporting of Observational Studies in Epidemiology Statement to design, conduct, and report our study.
Inclusion criteria were being age ≥18 y, having COVID-19 pneumonia with hypoxemia (ie, requiring at least 4 L/min of oxygen through a nasal cannula), and requiring at least one PP session. Subjects requiring noninvasive or invasive mechanical ventilation and/or mechanical circulatory assistance (extracorporeal membrane oxygenation) and subjects with poor echogenicity were excluded. Hemodynamic and respiratory responses to PP were simultaneously assessed by blood gas analysis and transthoracic echocardiography. Oxygen was administered using Hamilton-S1 (Hamilton Medical, Bonaduz, Switzerland) or Evita XL ventilators (Dräger, Lübeck, Germany) via a high-flow nasal cannula or through a face mask or a standard nasal cannula together with a humidification system Aquapak (Teleflex, Wayne, Pennsylvania).
Data Collection
We collected medical history and anthropometric data such as weight and height to calculate the body surface area and body mass index. PP lasted 1–3 h. All hemodynamic and respiratory data were recorded immediately before PP (T1 SP1), immediately before return to SP (T2 PP), and during the first hour following the return to SP (T3 SP2). No hemodynamic interventions (fluid boluses or vasoconstrictors) were performed from T1 SP1–T3 SP2. We also collected breathing frequency and continuous SpO2 data at these time points. Blood gases were sampled via an indwelling arterial catheter to assess PaO2, PaCO2, and pH. FIO2 values were collected from the medical record. The FIO2 value used to calculate PaO2/FIO2 was calculated in case high-flow oxygen delivery was used according to the following rule: FIO2 = 21 + 2.5 × oxygen flow (L/min).16
We performed a transthoracic echocardiography (Vivid S6, GE Healthcare, Madison, Wisconsin) at T1 SP1, T2 PP, and T3 SP2. The comprehensive echocardiographic examination in SP before PP consisted of apical 4-chamber and 2-chamber views, parasternal long- and short-axis views, and transhepatic view of the vena cava and portal vein with Doppler and tissue Doppler analysis. For most subjects, we were able to put the probe exactly at the same spot in prone and SP for the 4-chamber apical view. During PP, apical and transhepatic views of the vena cava and portal vein only were performed. Left ventricular (LV) outflow tract (LVOT, average of 2 measurements) and LVOT velocity time integral (VTI) (LVOT VTI, average of 5 measurements in case of sinus rhythm and 10 in case of arrhythmia) were recorded. LVOT surface was calculated as follows: 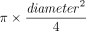 . The stroke volume index (SVI) was calculated as
. The stroke volume index (SVI) was calculated as 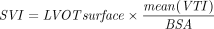 and CI as
and CI as 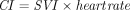 . We also estimated the LV end-diastolic and end-systolic volumes and the LV ejection fraction using the Simpson biplane method, the RV end-diastolic and end-systolic areas (RVEDA and RVESA), the fractional area changes (FACs) defined as
. We also estimated the LV end-diastolic and end-systolic volumes and the LV ejection fraction using the Simpson biplane method, the RV end-diastolic and end-systolic areas (RVEDA and RVESA), the fractional area changes (FACs) defined as 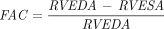 , the tricuspid annular plane systolic excursion (TAPSE), the tricuspid S′, the mitral and tricuspid E/e′ ratio, and the venous portal pulsatility. RV dilation was defined from the diameters and lengths of the right ventricle according to the definition of the guidelines of chamber quantification.17 We collected the portal venous pulse-wave Doppler flow, assessed using a probe position in the mid to posterior axillary position in a sagittal hepatic approach. Portal pulsatility was defined as
, the tricuspid annular plane systolic excursion (TAPSE), the tricuspid S′, the mitral and tricuspid E/e′ ratio, and the venous portal pulsatility. RV dilation was defined from the diameters and lengths of the right ventricle according to the definition of the guidelines of chamber quantification.17 We collected the portal venous pulse-wave Doppler flow, assessed using a probe position in the mid to posterior axillary position in a sagittal hepatic approach. Portal pulsatility was defined as 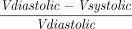 . Capillary refill time (CRT) was measured 3 times using a chronometer after firm compression of the index for 7 s. The average of the 3 measurements was then calculated and analyzed. An independent assessment of the echocardiographic CI was performed by the 2 principal investigators (ZR and MJL). The ratio of oxygen saturation (ROX) index18 was calculated as
. Capillary refill time (CRT) was measured 3 times using a chronometer after firm compression of the index for 7 s. The average of the 3 measurements was then calculated and analyzed. An independent assessment of the echocardiographic CI was performed by the 2 principal investigators (ZR and MJL). The ratio of oxygen saturation (ROX) index18 was calculated as  .
.
The severity status was evaluated using the Simplified Acute Physiology Score (SAPS II)19,20 and Sequential Organ Failure Assessment score.21,22
Statistics
Sample size calculation was based on our main objective, that is, to compare the CI before, during, and after PP. Taking into consideration the results of Jozwiak et al,6 we hypothesized that an increase of 10% in CI in the overall population could be expected. Estimating an average CI of 3.0 L/min and an overall SD 0.5, we calculated an effect size of 0.6. Using Cohen method, we calculated that 24 subjects were needed.23 We used a Shapiro-Wilk test and QQ plot to assess the normal distribution of the data. We computed the coefficient of variation (mean divided by SD) to assess the inter-observer and intra-observer reproducibility for the VTI. The precision corresponded to 2 times the coefficient of variation. The least significant change (LSC) of the CI was the minimum change to differentiate a true change of CI from random variation. The LSC was computed as follows: 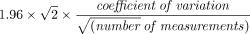 . Paired and non-paired 2-tailed Student t test or Wilcoxon test was used to compare the characteristics of subjects before and after PP, as well as between those for whom the CI significantly increased versus did not during the PP session. The threshold of the significant increase in CI was set at 15%.24 For categorical data, the Fisher exact test and a chi-square test were used appropriately. We assessed the absence of deviation from homoscedasticity or normality by visual inspection of residual plots. Comparison between the different steps of the study was done using analysis of variance with repeated measures. Bonferroni correction was used to correct multiple testing for post hoc analysis in repeated measures. Missing data were handled by pairwise deletion for each variable. We computed descriptive and analytical statistical analysis using the Free Software Foundation’s CRAN R (R Foundation for Statistical Computing, Vienna, Austria). Tests were 2-sided. P < .05 was considered statistically significance.
. Paired and non-paired 2-tailed Student t test or Wilcoxon test was used to compare the characteristics of subjects before and after PP, as well as between those for whom the CI significantly increased versus did not during the PP session. The threshold of the significant increase in CI was set at 15%.24 For categorical data, the Fisher exact test and a chi-square test were used appropriately. We assessed the absence of deviation from homoscedasticity or normality by visual inspection of residual plots. Comparison between the different steps of the study was done using analysis of variance with repeated measures. Bonferroni correction was used to correct multiple testing for post hoc analysis in repeated measures. Missing data were handled by pairwise deletion for each variable. We computed descriptive and analytical statistical analysis using the Free Software Foundation’s CRAN R (R Foundation for Statistical Computing, Vienna, Austria). Tests were 2-sided. P < .05 was considered statistically significance.
Results
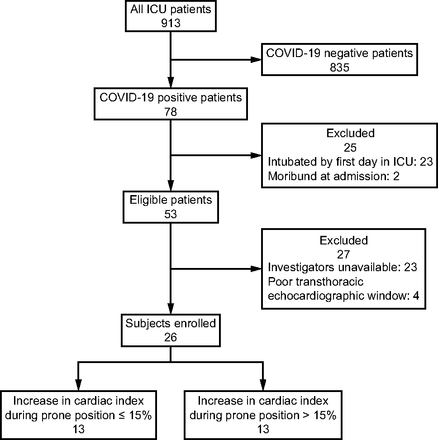
A total of 26 subjects were included in the present study (Fig. 1). There were 19 (73%) male subjects; the mean ± SD body mass index was 29 ± 6; the median [interquartile range, IQR] age was 70 [58–74] y, and the mean ± SD initial SAPS II was 40 ± 12. We used predominantly high-flow nasal oxygen therapy (22, 85%) during PP (Table 1). Hemodynamic failure was rare, and norepinephrine was only used for 2/26 (7.5%) subjects, and no subject required inotropic agents. The mortality at day 28 was 35% (9/26 subjects). Subjects were classified according to the increase in CI during PP: 13 (50%) experienced an increase > 15% and 13 (50%) an increase ≤ 15%. Subjects for whom the increase was > 15% had lower initial CI values and tended to have lower volumetric markers of preload. They also had lower markers of RV systolic function at baseline (Table 1).
Flow chart of the study. CI = cardiac index; PP = prone positioning.
Baseline and Clinical Characteristics
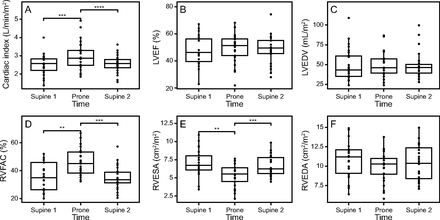
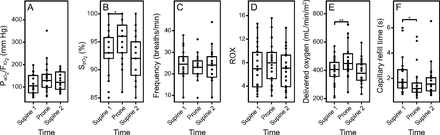
Regarding the main objective, we found a 20% increase in CI during PP: T1 SP1 2.5 ± 0.6 L/min/m2, T2 PP 3.0 ± 0.8 L/min/m2, and T3 SP2 2.6 ± 0.5 L/min/m2, P < .001. Accordingly, SVI increased from 32 ± 6 mL/m2 at T1 SP1 to 37 ± 9 mL/m2 during PP and dropped to 33 ± 7 mL/m2 at T3 SP2 (P < .001). We also observed a significant increase in both RVFAC (T1 SP1 36 ± 10%, T2 PP 46 ± 10%, and T3 SP2 35 ± 8%, P < .001) and TAPSE (T1 SP1 18 ± 6 cm, T2 PP 22 ± 6 cm, and T3 SP2 20 ± 5 cm, P = .007) in PP and a significant reduction of RVEDA and portal pulsatility (Fig. 2). PP led to a significant increase in SpO2 but not in the PaO2/FIO2. We also observed a significant increase in oxygen delivery and a significant reduction of the CRT during PP (Fig. 3).
Echocardiographic hemodynamic evolution during a prone position session. CI = cardiac index; ANOVA = analysis of variance; SP1 = supine position 1 (before PP); PP = prone positioning; SP2 = supine position 2 (after PP); PWC = pairwise comparison; LVEF = left ventricular ejection fraction; LVEDV = left ventricular end-diastolic volume; RVFAC = right ventricular fractional area change; RVESA = right ventricular end-systolic area; RVEDA = right ventricular end-diastolic area.
Respiratory and peripheral perfusion variables before, during, and after prone positioning. ANOVA = analysis of variance; SP1 = supine position 1 (before PP); PP = prone positioning; SP2 = supine position 2 (after PP); PWC = pairwise comparison; ROX = ratio of oxygen saturation; DO2 = oxygen delivery; CRT = capillary refill time.
Regarding the correlation between the CI and RV function, we found a significant correlation between FAC and CI (r2 = 0.14, P < .001). We also found a significant correlation between FAC variations and CI variations (r2 = 0.26, P = .011; variations were determined between before and after PP).
Regarding reproducibility, for 10 subjects, 5 VTI measurements were collected and traced 2 times by ZR and once by MJL. The coefficient of variation [95% CI] for the intra-observer variability was 3.18% [2.40–3.94] and 7.8% [5.8–9.8] for the intra-observer variability for a single VTI. The LSC as the average of 5 measurements of the VTI in the whole cohort was 9.9% [7.3–12.4].
Discussion
In the present study including subjects with COVID-19 acute respiratory failure, awake PP induced an increase in CI associated with a significant improvement in RV systolic function. This improvement was reversible when subjects returned to SP and could be explained by the reduction in RV afterload, as the RV end-systolic surface decreased. Indeed, RV function is sensitive to afterload, and a decrease in afterload induces an increase in stroke volume mediated by the reduction of the RV end-systolic volume.25 Our findings are consistent with those of Jozwiak et al6 who have reported a reduction in pulmonary vascular resistance related to the reduction of hypoxemic pulmonary vasoconstriction in subjects for whom the CI increases during PP.
Furthermore, the increase in CI could be responsible for an improvement in peripheral perfusion, as suggested by the significant decrease in CRT and a significant increase in oxygen delivery during PP. This could, however, be the consequence of a reduction in venous congestion, as suggested by the significant decrease in portal pulsatility during PP due to increased venous return. Portal pulsatility is a marker of venous congestion or RV diastolic function, and a reduction in afterload induced by pulmonary vasodilators has been shown to reduce portal pulsatility.26 Conversely, an increase in portal pulsatility has been associated with an increase in lactate level.27 It has also been associated with kidney dysfunction and liver cytolysis.26-30 This last point could be of interest when assessing the potential benefit of awake PP in the context of acute respiratory failure since a quarter of patients with COVID-19 acute respiratory failure develop acute kidney injury,31 the latter being associated with a high mortality rate, especially when renal replacement therapy is required. It is unclear in the present study whether an increase in preload could have played a role in CI increase, as previously reported for ventilated subjects.6 Subjects who experienced an increase in CI tended to display lower markers of preload at baseline (less RV dilation and lower left end-diastolic volume or portal pulsatility), making them more susceptible to an increase in CI in case of preload increase. They also had poorer RV systolic function, making them more likely to increase CI because of a reduction in afterload. We also found a significant correlation between CI variations and FAC variations during the change from supine to prone position. This supports the causal mechanism of an increase in CI led by an increase in RV function.
Considering our data, we suggest that future fields of clinical research investigating PP should focus on hemodynamic effects. Subjects with diastolic or systolic acute RV dysfunction with mild-to-moderate hypoxemia could be tested to further assess the effects of PP in this high-risk population.
Respiratory parameters in the context of acute respiratory failure are not the only issue to be considered, and cardiac function should also be considered, as in our study > half of the subjects had a low CI or RV dilation at baseline.
Regarding respiratory parameters, we only found a significant increase in SpO2 and a trend toward an increase in the PaO2/FIO2. This could be explained by the lack of power of our study, but it also suggests the hemodynamic effects of PP are greater than the respiratory effects. As a decrease in the CI can improve the PaO2/FIO2 through a PEEP trial, an increase in CI could mitigate the increase in PaO2/FIO2 during PP.32 In fact the increase in CI can result in an increase of the shunt fraction evaluated by the pulmonary shunt, leading to a less significant increase in lung oxygenation.33
The present study relies on several strengths: Awake PP is a simple technique that can be carried out in any medical facility. We precisely assessed intracardiac hemodynamics with the help of comprehensive echocardiography and evaluated peripheral perfusion. We checked the reproducibility of our measurements by assessing both the inter- and intra-reproducibility and found a LSC of CI variation < the significant change observed during PP, which clearly reinforces the internal validity of the study. The overall hemodynamic coherence of our results (ie, the increase in CI, systolic RV function enhancement, and peripheral perfusion improvement) is another key point for the biological plausibility and the internal validity of our findings. Our 3-step approach including data acquisition after return to SP and showing that most hemodynamic variables returned to baseline values reinforce the probability that changes can be explained by PP alone and decrease the probability of a time effect.
To investigate the mechanism of CI during PP, we divided the cohort into a group with significant CI increase and one with a non-significant CI increase. The threshold to define a significant increase was set at 15%. This threshold is often chosen to define a response to fluid; due to the VTI LSC of transthoracic echocardiogram (TTE), it has been conclude that TTE could detect a 15% change in CI.24 The LSC found in our study supports this threshold as the LSC and its CI are < 15%.
We must, nonetheless, acknowledge some limits of the study. First, our study lacked power regarding an improvement of respiratory parameters. Second, one can argue that thermodilution is a better clinical standard to assess CI at the bedside, but using pulmonary arterial catheter or transpulmonary thermodilution was considered too invasive for awake patients. We also intended to collect tricuspid regurgitation gradient, but due to missing data, this variable was not analyzed. Similarly, central venous pressure would have been helpful to further assess the potential reduction in venous congestion suggested by portal pulsatility changes. As the reduction in portal pulsatility is associated with an improvement in kidney and liver functions, it would have been convincing to assess them during PP, but longer periods of PP would have been needed. Eventually, it would have been more accurate to assess preload dependence with a passive leg raising before PP to confirm the hypothesis of a preload reserve to explain the increase in CI.
Conclusion
PP improved CI and RV systolic and diastolic functions in awake subjects with COVID-19 with acute respiratory failure and without mechanical ventilation support. This improvement could be explained mainly by the reduction in RV afterload as assessed by the reduction of RV systolic area. The present study allows a better understanding of the beneficial effects of awake PP, which are not limited to respiratory function but also concern RV function. Further studies may include the assessment of hemodynamic changes in regard to kidney and liver function.
Footnotes
- Correspondence: Matthias Jacquet-Lagrèze MD PhD, Service d’anesthésie-réanimation, Hôpital Louis Pradel, Hospices Civils de Lyon, 59, Boulevard Pinel, 69394 Lyon Cedex, France. E-mail: matthias.jacquet-lagreze{at}chu-lyon.fr
See the Related Editorial on Page 852
Dr Jacquet-Lagrèze is cofounder and shareholder of DiCARTECH, a company created to build and sell a device that measures the capillary refill time. Dr Jacquet-Lagrèze discloses relationships with Baxter, Edwards, and Dräger.
- Copyright © 2023 by Daedalus Enterprises