Abstract
Airway pressure-release ventilation (APRV) is used in the management of patients with severe or refractory respiratory failure. In addition to reversal of inspiratory-expiratory ratios, this pressure control mode also allows unrestricted spontaneous breathing. The spontaneous tidal volume (VT), as well as the VT resulting from transition between the high and low airway pressures, is uncontrolled. There are limited data on the within-patient variation of actual VT and the safety of these modes. The authors present a patient with severe ARDS who was managed with biphasic modes (APRV and bi-level positive airway pressure). Serial VT measurements showed that VT ranged from 4 to 12 mL/kg predicted body weight. Computed tomography scan images and chest radiographs obtained before and following APRV showed lung parenchyma changes that may be related to ventilator-induced lung injury. We also present a mathematical model that is useful for simulating APRV and demonstrating the issues related to volume delivery for mandatory breaths during the transition between the 2 pressure levels. A key finding of this analysis is the interdependence of release volume, autoPEEP, and the Tlow time setting. Furthermore, it is virtually impossible to target a specific PaCO2 with a desired level VT and autoPEEP in a passive model, emphasizing the importance of spontaneous breathing with this mode. This case report suggests caution when using these modes, and that end-inspiratory lung volumes and VT should be limited to avoid lung injury. The important point of this case study and model analysis is that the application of APRV is more complex than it appears to be. It requires a lot more knowledge and skill than may be apparent from descriptions in the literature.
- airway pressure release ventilation
- bi-level
- BPAP
- mechanical ventilation
- ventilator-induced lung injury
- ventilator associated lung injury
- volutrauma
- atelectrauma
Introduction
Airway pressure release ventilation (APRV) is a form of pressure control intermittent mandatory ventilation (PC-IMV) typically used in the setting of acute lung injury and severe hypoxemia.1 During APRV, airway pressure is set at 2 levels, sometimes called Phigh and Plow, for 2 time periods, called Thigh and Tlow. These are analogous to inspiratory pressure, PEEP, inspiratory time, and expiratory time, respectively. Two unconventional features of APRV are:
Extreme inverse inspiratory-expiratory ratio (eg, Thigh = 4 s and Tlow = 0.5 s)
Use of an active exhalation valve that allows unrestricted spontaneous ventilation to occur during Thigh as well as Tlow
There are few clinical trials showing that APRV is able to provide improved gas exchange, and it is unclear if APRV results in better clinical outcomes or can worsen lung injury.2 Particularly, the combination of a pressure control mode and the ability to breathe spontaneously can result in unpredictable tidal volume (VT). Consistently, high VT has been associated with lung injury and worse outcome in patients with3 and without4,5 ALI/ARDS. We report our experience with a patient in whom APRV was used in the setting of severe, refractory hypoxemia, and imaging studies were available before and after the onset of acute illness.
Case Report
A 20-year-old, previously healthy patient, presented to an outside facility with 3 day history of flu like symptoms, including fever, stuffy nose, and cough, with production of purulent sputum. He also reported right upper quadrant pain and vomiting on the day of admission. At admission he was found to have leukocytosis and abnormal liver function tests. Chest computed tomography (CT) scan performed the day following admission showed a small right pleural effusion and infiltrates in the right upper and lower lobes.
The patient was endotracheally intubated on the third day following admission, for laparoscopic surgery. Bronchoscopy performed at that time revealed purulent infiltrates in the right lower lobe. He was extubated following the procedure but developed respiratory distress, and on the day following surgery required endotracheal intubation for mechanical support using pressure control continuous mandatory ventilation (PC-CMV). Respiratory failure progressed rapidly over the next 72 hours, with bilateral diffuse infiltrates evident on radiographs along with severe hypoxia requiring FIO2 of 1.0 and PEEP of 14 cm H2O to maintain oxygenation. On the third day following intubation for respiratory failure, the patient was transferred to our facility. At that time he was ventilated using PC-CMV (PB 840 ventilator, Covidien, Boulder, Colorado) with an inspiratory pressure (above PEEP) of 24 cm H2O, PEEP = 14 cm H2O, and FIO2 = 1.0. The patient was paralyzed using continuous infusion of vecuronium, and sedated with propofol and lorazepam.
During transfer the patient developed a brief period of hypoxemia, to an oxygen saturation of 68%, which responded to manual ventilation. Following arrival at our institution, the patient continued to be hypoxemic, and a decision was made to switch to a non-conventional mode of ventilation, and neuromuscular blockade was discontinued. Bi-level positive airway pressure (BPAP) ventilation (named “BiLevel” on the PB 840 ventilator) was initiated using Phigh = 35 cm H2O and Plow = 15 cm H2O (inspiratory pressure6 = 20 cm H2O). Spontaneous breaths during Phigh were not assisted with pressure support. On these ventilator settings, VT (extracted from the electronic medical record) was documented as varying between approximately 450 mL and 600 mL (7 mL/kg to 9 mL/kg for ideal body weight = 69 kg). This variation in VT was attributed primarily to the patient's changing inspiratory efforts, which continued throughout the course of mechanical ventilation.
Within a few hours the BPAP settings were changed to Phigh = 30 cm H2O and Plow = 5 cm H2O (inspiratory pressure = 25 cm H2O). On these ventilator settings, VT varied between approximately 11 mL/kg and 12 mL/kg from day 2 to day 3. From day 4 to day 8, the VT variation increased, with values ranging from about 5 mL/kg to 12 mL/kg.
On the 8th day following transfer to our institution, a decision was made to switch to APRV mode, with short Tlow and exhalation to 0 PEEP. Settings were Plow = 0 cm H2O, Phigh = 25 cm H2O (inspiratory pressure = 25 cm H2O), Thigh = 6.4 s, and Tlow = 0.3 s, which resulted in a mandatory breath frequency of 9 breaths/min. When the patient was switched to APRV, the VT ranged from approximately 4 mL/kg to 12 mL/kg).
Figure 1 shows the time course from measured values of VT and airway pressure. Table 1 summarizes the ventilator modes associated with the VT observed during the course of mechanical ventilation.
Time course of mechanical ventilation, showing the tidal volumes that resulted from settings for inspiratory pressure (Phigh) and end-expiratory pressure (Plow).
Summary of Ventilator Settings and Tidal Volume Ranges
Comparison of CT scans from admission and day 12 shows extensive ventilator-associated lung injury (Fig. 2). Serial chest radiographs show the progression of lung damage (Fig. 3). A week later the patient underwent a tracheostomy procedure and was gradually weaned from mechanical ventilation. Twenty-four days after his transfer, the patient was discharged to a skilled nursing facility for occupational therapy and physical therapy.
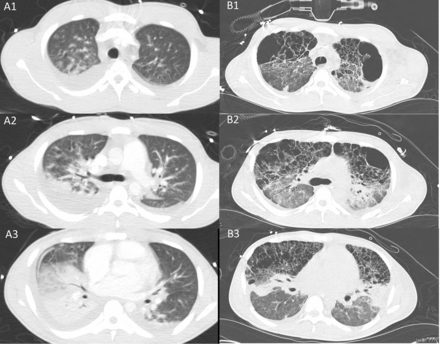
Representative sections from computerized tomographic images of the chest. Series A1–3 were obtained 2 days after initial onset of flu like symptoms. Series B1–3 were obtained 17 days later. Series A1–3 show typical features of ARDS, with consolidation predominantly in dorsal regions of the lung and bilateral pleural effusions. Seventeen days later, volutrauma is evident, predominantly in the ventral portions of the lung. This suggests that the consolidated portions of the lung were protected from injury due to high volumes.
Representative chest radiographs that show the evolution of ventilator-induced lung injury. On days 0 and 5, when the patient was on pressure control continuous mandatory ventilation, there is no evidence for ventilator-induced lung injury. However, hyperinflation of the left lung is evident. On day 8, after 3 days of ventilation in APRV/BiLevel, areas of hyperlucency are evident on both sides. By day 15, cystic changes are evident (open arrows). Day 25 shows progression of lung injury with pneumothorax on the lower right side (3 arrows).
Discussion
In the simplest terms, the goals of mechanical ventilation are to promote safety, comfort, and liberation.7 The goal of safety includes the objectives of optimizing both gas exchange and the pressure-volume relation of the lungs. The latter objective implies that mean lung volume is adjusted such that compliance is maximized. Mean lung volume is a function of both the end-expiratory lung volume (eg, by setting “optimal” PEEP) and VT. VT is the only variable shown to directly affect long-term outcomes in mechanically ventilated patients.8 In particular, for patients with ARDS, the objective should be to keep VT within approximately 6–8 mL/kg, with larger VT presumably increasing morbidity and mortality. For this reason, some clinicians prefer volume control modes because VT is naturally more variable with pressure control modes. As a form of pressure control, both APRV and BPAP involve a number of factors that may increase VT variability, compared to other modes in this category. Indeed, Kallet has observed that “Of the APRV studies that have measured release volumes, mean volumes have been reported between 550 mL to 840 mL and 9 mL/kg by measured body weight, which probably translates into 11 mL/kg predicted body weight. In many studies these values exceeded current lung-protective ventilation targets.”9
We will now review the factors contributing to VT variability as they relate to our case study. Some authors assert that “Rather than generating a tidal volume by raising the airway pressure above the set PEEP, release volumes in APRV are generated by briefly releasing airway pressure from Phigh to Plow. Because ventilation with APRV results as airway pressure and lung volume decrease, the risk of over-distention may be reduced.”10 The common description of biphasic modes as “2 levels of CPAP” is misleading, as it obscures the fact that the transition from Plow to Phigh and from Phigh to Plow constitutes a mandatory breath (ie, inspiration is machine triggered and machine cycled7), just as in any other form of PC-IMV. We have observed that when clinicians fail to recognize that mandatory breaths are being delivered, they also overlook the associated VT and tend to focus only on the spontaneous breathing activity of the patient. They also seem to think that because the patient spends the majority of the time at Phigh, adequate lung volume is being maintained and the brief “pressure releases” are inconsequential in terms of lung derecruitment.
The data from this case study suggest that such ideas are indeed misconceptions. Ventilator-associated lung injury is generally thought to occur both as a result of repetitive collapse and reopening of lung units due to inadequate end-expiratory pressure (atelectrauma) and as the result of stretch injury due to excessive end-inspiratory volumes (volutrauma).11 APRV in this patient resulted in unknown levels of end-expiratory pressure and VT that was higher than the generally accepted target of 6 mL/kg for patients with ARDS. The CT scans suggest that severe ventilator-associated lung injury occurred. Representative chest radiographs (see Fig. 3) show the evolution of ventilator-induced lung injury (VILI). There is no evidence for VILI from days 0–5, when the patient was on PC-CMV. However, hyperinflation of the left lung is evident. On day 8, after 3 days of ventilation in APRV/BiLevel, areas of hyperlucency are evident on both sides. Cystic changes are evident by day 18 (open arrows) and progression is evident by day 25, with pneumothorax on the right side.
The culture of the purulent infiltrates obtained from bronchoscopy were negative, with many epithelial cells. While necrotizing pneumonia can present with cavitary lung disease, the CT appearance is very distinctive. Also note that the cystic disease involved the non-consolidated areas of the lung and hence is pathognomonic of VILI. We do not believe that APRV “caused” VILI in this patient: only that it was associated with a large variation in VT, and that the VT was large enough to potentially contribute to volutrauma. Although our data cannot be used to imply causation, we can certainly call into question the “lung-protective” features of APRV when no lung-protective parameters (ie, end-expiratory pressure and VT) are explicitly monitored and managed.
The transpulmonary pressure difference (pressure at the airway opening minus pressure in the pleural space) determines lung volume change and is usually not monitored during mechanical ventilation. Reduced thoracic and abdominal compliance in critically ill patients may require higher airway pressure to achieve the transpulmonary pressure difference that provides acceptable volume change.12 Spontaneous breathing efforts, however, superimpose marked variability in transpulmonary pressure and therefore VT. Some ventilators allow the assistance of spontaneous breaths using pressure support. If used, this additional assistance may not only further increase VT variability but will alter the relationship between APRV settings and expected PaCO2, because it affects the proportion of work performed by the patient versus the ventilator. Setting APRV pressure targets to predefined arbitrary limits does not guarantee VT limitation, as documented by our case report. In addition, the fortuitous availability of imaging studies in this patient indicates that the higher VT resulting from the large transpulmonary pressure differences may not necessarily result in improvement of dependent lung, but, rather, in over-distention and volutrauma.
Practical Implications of Case Study
The term APRV, first described by Stock et al,1 is used loosely in the literature and often confused with biphasic positive airway pressure (BIPAP), as first described by Baum et al.13 Both are classified14 as pressure control intermittent mandatory ventilation (PC-IMV) (ie, mandatory breaths that are time triggered, pressure targeted, and time cycled, with spontaneous breaths possible during and between mandatory breaths). Some adult ventilators (notably those made by Dräger) and almost all infant ventilators have always allowed unrestricted spontaneous breathing during mandatory pressure control breaths, while others have not. Newer ventilators have added APRV capability to their list of modes, under various proprietary names, such as “BiLevel” (Covidien PB 840), “Airway Pressure Release Ventilation” (Dräger Evita XL and Hamilton G5), “Duo Positive Airway Pressure” (Hamilton G5), and “Bi-Vent” on the Maquet Servo-i. Ironically, you can now find within a single ventilator both a mode that is PC-IMV with spontaneous inspiration (but not expiration) permitted during mandatory breaths, and PC-IMV with unrestricted spontaneous breathing (inspiration and expiration) during mandatory breaths (eg, PC-IMV vs BiLevel on Covidien PB 840).
The difference between BIPAP and APRV is in the timing of the upper and lower pressure levels. In BIPAP, Thigh is usually shorter than Tlow.15 Therefore, in order to avoid derecruitment, Plow has to be set above zero. Rose et al found that, compared to BIPAP, APRV was described more frequently as extreme inverse inspiratory-expiratory ratio and used rarely with non-inverse ratios. One BIPAP and 8 APRV studies used mild inverse ratio (1:1 to 2:1). There was increased use of 1:1 ratio with BIPAP. In adult studies, the mean Phigh was 6 cm H2O greater with APRV than with BIPAP. For both modes, the mean reported Plow was 5.5 cm H2O.16 To make things even more confusing, the term BiPAP is used on Philips Respironics ventilators to signify pressure control continuous spontaneous ventilation (PC-CSV) (ie, breaths are patient triggered, pressure targeted, and flow cycled, also known as pressure support). We prefer the term “biphasic” as a generic name to distinguish pressure control modes with unrestricted spontaneous breathing during mandatory breaths from conventional PC-IMV and PC-CSV.
Unlike conventional PC-CMV on most adult ventilators (often called “pressure control mode”), APRV accommodates the patient's breathing pattern and allows superimposition of spontaneous breathing on mandatory breaths.17 Peak airway pressure in APRV does not exceed the set level, and spontaneous breathing efforts augment minute ventilation. One of the important goals of APRV is to promote spontaneous breathing. A theoretical benefit of allowing spontaneous ventilation to occur during mechanical ventilation is to preserve diaphragmatic activity and therefore ventilation to the dependent areas of the lung.18,19
Theoretical Analyses Using a Mathematical Lung Model
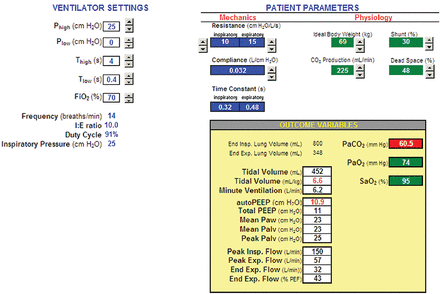
The practice of setting Plow to zero and relying on autoPEEP to maintain end-expiratory lung volume deserves some consideration. Proponents of APRV recommend Tlow values in the range of 0.2–0.8 seconds10,12 to achieve adequate autoPEEP, although in practice autoPEEP is virtually impossible to measure when the patient is making active inspiratory efforts. What has not been adequately addressed in the literature is the difficulty of managing ventilatory parameters due to the interdependence of autoPEEP and mandatory breath tidal volumes (ie, the tidal volumes resulting from the transition of Plow to Phigh and vice versa). To demonstrate this, we developed a spreadsheet based model (Fig. 4, free download available at http://www.mediafire.com/view/?23psqtqhc58pb88) using the equations governing pressure control ventilation developed by Marini et al20 (Table 2). The model simulated a ventilator with APRV settings connected to a patient with lung mechanics that might be observed in patients with ARDS.21 The ventilator settings were: Phigh = 25 cm H2O, Plow = 0 cm H2O, Thigh = 4 seconds, Tlow = 0.2 seconds to 0.8 seconds. The patient (ideal body weight = 69 kg) was modeled by inspiratory resistance = 10 cm H2O·s/L, expiratory resistance = 15 cm H2O·s/L, and compliance = 32 mL/cm H2O, with no inspiratory effort. Dead space as a percentage of VT was arbitrarily set at 48%. PaCO2 was simulated using the equation22:
Patient-ventilator simulator implemented with a spreadsheet (see text for explanation).
Equations for Mathematical Model of Pressure Control Ventilation
The calculated values for autoPEEP (equivalent to total PEEP or end-expiratory lung pressure because set PEEP was zero), VT (from mandatory breaths), and simulated PaCO2 were plotted against values of Tlow. Figure 5 shows the interdependence of autoPEEP and VT. AutoPEEP ranged from 4.7 cm H2O (Tlow = 0.8 s) to 16.5 cm H2O (at Tlow = 0.2 s). Thus, at some Tlow settings, end-expiratory lung pressure is unlikely to provide “optimum PEEP” for a patient with ARDS,25 particularly given the presumption that the lung is recruitable, and thus it is appropriate for the patient to spend most of his time at a relatively high “CPAP” level (eg, 20–35 cm H2O).10,12 We concede that the meaning of “optimum PEEP” is debatable.26 Consideration of appropriate end-expiratory pressure/lung volume is critical, given the general acceptance of the idea that cyclical alveolar opening and closing is injurious to patients with acute lung injury or ARDS.27 VT ranged from 4.0 mL/kg (Tlow = 0.2 s) to 9.4 mL/kg (Tlow = 0.8 s). In this simulation, Tlow values above 0.4 s resulted in VT greater than the generally accepted safe upper limit of 6 mL/kg.8 Simulated PaCO2 (Fig. 6) ranged from 46.0 mm Hg (Tlow = 0.8 s) to 95.9 mm Hg (at Tlow = 0.2 s). The values expected for PaCO2 in a real patient would be less, depending on how much spontaneous minute ventilation the patient could generate. However, the VT range would be greater, due to the contribution of inspiratory muscle pressure change to the ventilator's inspiratory driving pressure (Phigh − Plow), assuming the use of a ventilator that synchronized mandatory breaths with spontaneous efforts (eg, PB 840 and Evita XL).
This figure illustrates the interdependence of autoPEEP, tidal volume, and the Tlow setting.
This figure illustrates the dependence of simulated PaCO2 on the Tlow setting.
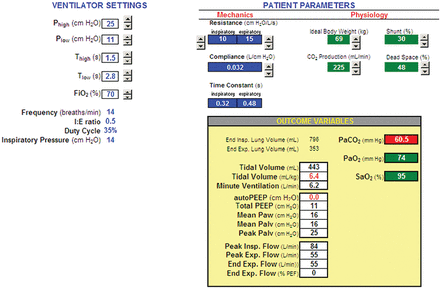
This simulation demonstrates the difficulty of applying APRV with zero Plow. That is, we find it virtually impossible to target a specific PaCO2 with a desired level VT and autoPEEP in a passive model. What this implies is that APRV is not a good choice for full ventilatory support. Granted, APRV is intended for partial support, but this exercise indicates the extent to which patients must regulate their own PaCO2 with spontaneous ventilation. Furthermore, the instantaneous values of VT and autoPEEP are even more unpredictable when factoring in spontaneous breathing efforts and the effect on the total system (ie, patient and ventilator) time constant of the resistance of the ventilator's expiratory manifold. Data from our lab show that there is a wide variance between the expiratory flow curve predicted by a mathematical model and actual flow curves using different ventilators.28 Furthermore, the patient's time constant changes substantially (by increasing resistance) with accumulation of secretions in the airways. Airway resistance can easily double by the time the patient shows obvious signs of the need for suctioning. Also, the respiratory system time constant is affected by changes in lung and chest wall compliance. In particular, in the absence of paralysis, transient (and unpredictable) changes in chest/abdominal muscle tension may decrease chest wall compliance, adding further uncertainty to this clinical problem. All of this might argue against APRV (with Plow = 0) in favor of BIPAP (with Plow set to optimal PEEP), because with the latter we can achieve relative independence of VT from Tlow, provided that Tlow is more than about 3 expiratory time constants. Figure 7 shows that by using a BIPAP strategy it is possible to obtain the same level of ventilation with the same VT and peak inspiratory pressure (as in Fig. 4) as APRV by simply setting Plow to the level of autoPEEP and decreasing Thigh. What you lose is mean airway pressure, which, in this example, decreases from 23 to 14.5 cm H2O. On the other hand, you can keep the same mean airway pressure by increasing Thigh (Fig. 8), but now the patient has to make up for the decrease in minute ventilation caused by the reduction in mandatory breath frequency from 14 to 5 breaths/min. So the tradeoff, to simplify, is between safety (predictable VT and end-expiratory pressure) and comfort (ie, patient work of breathing). Clearly, these issues require further study.
Simulated ventilator with BIPAP setting (inspiratory-expiratory ratio < 1:1) with the same end-expiratory lung pressure, tidal volume, and alveolar ventilation, but lower mean airway pressure, compared to APRV in Figure 4.
Simulated ventilator with BIPAP setting (inspiratory-expiratory ratio < 1:1), with the same end-expiratory lung pressure, tidal volume, and mean airway pressure, but lower alveolar ventilation, compared to APRV in Figure 4.
In conclusion, we advise the reader not to draw conclusions regarding causation from a single case report. However, our findings and their theoretical underpinnings should alert users of biphasic modes to their potential complications. The important point of this case study is that the application of biphasic modes requires a lot more knowledge and skill than may be apparent from descriptions in the literature. When patients are mechanically ventilated using pressure control modes of ventilation that encourage superimposed spontaneous ventilation, such as with biphasic modes, we recommend that close attention be paid to VT, and when possible, autoPEEP. Respiratory drive, and hence spontaneous VT and autoPEEP levels, may be labile and depend on levels of sedation. This is especially important when ICU protocols implement daily awakening trials into their routine practice.
Footnotes
- Correspondence: Robert L Chatburn MHHS RRT-NPS FAARC, Respiratory Institute, A90-131, The Cleveland Clinic, 9500 Euclid Avenue, Cleveland OH 44195. E-mail: chatbur{at}ccf.org.
Dr Sasidhar has disclosed a relationship with Covidien. Mr Chatburn has disclosed relationships with Dräger, Hamilton, CareFusion, Covidien, Newport, IngMar, Radiometer America, Breathe Technologies, and the Alpha-1 Antitrypsin Foundation.
- Copyright © 2012 by Daedalus Enterprises Inc.