Abstract
Mechanically ventilated patients in respiratory failure often require adjunct therapies to address special needs such as inhaled drug delivery to alleviate airway obstruction, treat pulmonary infection, or stabilize gas exchange, or therapies that enhance pulmonary hygiene. These therapies generally are supportive in nature rather than curative. Currently, most lack high-level evidence supporting their routine use. This overview describes the rationale and examines the evidence supporting adjunctive therapies during mechanical ventilation. Both mechanistic and clinical research suggests that intrapulmonary percussive ventilation may enhance pulmonary secretion mobilization and might reverse atelectasis. However, its impact on outcomes such ICU stay is uncertain. The most crucial issue is whether aerosolized antibiotics should be used to treat ventilator-associated pneumonia, particularly when caused by multi-drug resistant pathogens. There is encouraging evidence from several studies supporting its use, at least in individual cases of pneumonia non-responsive to systemic antibiotic therapy. Inhaled pulmonary vasodilators provide at least short-term improvement in oxygenation and may be useful in stabilizing pulmonary gas exchange in complex management situations. Small uncontrolled studies suggest aerosolized heparin with N-acetylcysteine might break down pulmonary casts and relieve airway obstruction in patients with severe inhalation injury. Similar low-level evidence suggests that heliox is effective in reducing airway pressure and improving ventilation in various forms of lower airway obstruction. These therapies generally are supportive and may facilitate patient management. However, because they have not been shown to improve patient outcomes, it behooves clinicians to use these therapies parsimoniously and to monitor their effectiveness carefully.
- high-frequency percussive ventilation
- intrapulmonary percussive ventilation
- inhaled nitric oxide
- aerosolized prostacyclin
- aerosolized heparin
- metered-dose inhalers
- heliox
Introduction
Two primary goals of mechanical ventilation are to establish adequate arterial oxygenation and carbon dioxide excretion, and to assume the power of breathing either to normalize or minimize respiratory muscle work load. Generally, these goals can be accomplished with standard ventilator manipulations alone. However, critically ill patients in respiratory failure often require direct pulmonary delivery of drugs, as well as inhaled medical gases, in order to reverse the underlying pathology and/or to stabilize gas exchange. Also, other adjunctive therapies are used either to improve pulmonary mechanics or facilitate bronchial hygiene.
In this paper I will review various adjunctive therapies frequently incorporated during mechanical ventilation. These will include a review of mechanically enhanced secretion clearance techniques, as well as various aerosol delivery devices. This will be followed by reviews of: inhaled antibiotics for ventilator-associated pneumonia (VAP); selective pulmonary vasodilators to improve gas exchange and reduce pulmonary vascular resistance in patients with ARDS, or pulmonary hypertension; anti-coagulants/radical oxygen species scavengers in the treatment of ARDS from inhalation injury; and heliox for patients with respiratory failure from severe airway obstruction. Each therapy is discussed in terms of its rationale and level of evidence. This may assist clinicians in evaluating whether these therapies should be incorporated, and under what circumstances they should be considered.
Airway Clearance Techniques
Intrapulmonary Percussive Ventilation
Applying high-frequency oscillations to the airways enhances secretion clearance when interfaced with either spontaneous breathing1 or conventional mechanical ventilation.2 The most popular has been high-frequency percussive ventilation3 or intrapulmonary percussive ventilation (IPV).2 IPV has been the object of particular interest in inhalational injury,2,4,5 which is characterized by damaged mucociliary function, extensive sloughing of airway epithelial cells, and cast formation resulting in severe airway obstruction, atelectasis, and pneumonia. The mechanism by which IPV may promote pulmonary secretion clearance is its asymmetrical flow pattern, whereby expiratory flow exceeds inspiratory flow to propel secretions forward (Fig. 1).6 Animal models of excessive secretion production suggest that incorporating a higher peak expiratory to peak inspiratory flow ratio of approximately 4:1 (ie, a peak expiratory flow of 3.8 L/s and a peak inspiratory flow of 1.3 L/s) at a frequency of 14 Hz substantially increases the rate of secretion clearance and augments the effects of postural drainage.7
Representation of a high-frequency, asymmetrical flow pattern. Expiratory flows exceed inspiratory flow by a ratio of at least 4:1, so that pulmonary secretions sheared from the airway wall will tend to be propelled toward the central airways rather than deeper into the lung periphery.
Critically ill patients often have impaired secretion clearance for several reasons, including the effects of oxygen therapy on mucociliary function, hypersecretion from infection, impaired cough in the presence of an artificial airway, or muscle weakness. The effectiveness of coughing and propelling secretions forward is based upon several factors. Probably the most important is the gas-liquid interaction, whereby a gas stream flowing over a mucus layer creates shear forces.1 These forces loosen secretions from the airway wall and propel them either deeper into the lung periphery, or toward the central airways where they can be suctioned.
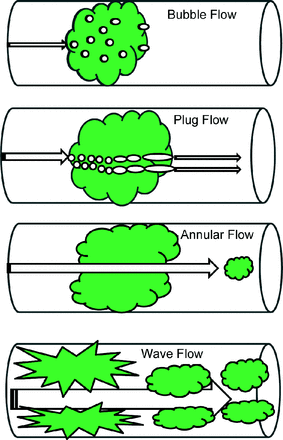
As mentioned above, this is determined by asymmetric flow relationships whereby peak expiratory flow must exceed inspiratory flow in order to propel secretions cephalad. This is referred to as “2-phase, gas liquid flow” and consists of 3 features in mucus-filled airways: bubble flow, plug flow, and annular flow (Fig. 2).8 Bubble flow occurs in the presence of mucus plugging, whereby low-flow gas passes through the obstruction as small bubbles. As airflow increases further, the bubbles grow in size and coalesce, breaking apart the mucus wall (plug flow), eventually creating a patent channel through the plug (annular flow). As gas flow velocity increases, the mucus layer becomes more unstable, first forming ripples and waves (wave flow) to a sufficient level that shear forces then peel away the mucus layer from the airway wall (as occurs during a forceful cough).8
Features of the “2-phase, gas liquid flow” mechanism by which mucus plugs are thought to be dislodged from the airways. Individual gas bubbles first penetrate the plug (bubble flow); as more penetrate the plug, these bubbles tend to coalesce into larger bubbles (plug flow) until they form a distinct channel through the plug, allowing for greater gas velocity (annular flow). As the gas velocity increases through the channel, so does the frictional force at the gas/liquid interface. This results in the development first of ripples and then waves across the mucus surface, until sufficient frictional or shear forces dislodge the plug from the airway wall (wave flow).
The rheologic characteristics of mucus also interact in complex ways with gas flow to determine mucus dynamics in the airways. These include the viscous and elastic properties of the mucus, as well as the thickness of the mucus layer.8 There is evidence suggesting that application of airway oscillations between 12–22 Hz for between 15–30 min acts as a “physical mucolytic” capable of altering the rheologic properties and promoting mobilization.9
Applying high-frequency oscillations either directly to the airway opening or to the chest wall appears to enhance secretion clearance, compared to no therapeutic intervention.10,11 However, most studies have not been done on patients requiring mechanical ventilation. Although comparisons between oscillatory devices and standard chest physical therapy (CPT) techniques have produced generally positive results, this has not yet been consistently demonstrated across all studies, at least in respect to outcome measures (Table 1). For example, in a large prospective randomized controlled trial (RCT), Clinkscale et al12 reported no difference between a traditional CPT regimen and chest wall oscillatory therapy in either ICU or hospital stay among adults with various pulmonary diseases.
Studies on IPV Examining Secretion Clearance and Reversal of Atelectasis in the ICU Setting
In contrast, several studies suggest that IPV may be superior to traditional CPT, particularly in regard to pulmonary hygiene. Toussaint et al1 reported significantly better secretion clearance (ie, a 40% increase in sputum weight) with IPV in a small randomized crossover trial in patients with Duchenne muscular dystrophy and excessive sputum production. Similar findings of significantly improved postoperative sputum production also were reported by Lucangelo et al13 when IPV was applied to patients undergoing pulmonary resection. Although total postoperative sputum production was not different between treatment groups, patients treated with IPV cleared a greater amount of secretions more rapidly (ie, 72% of their total sputum was cleared by the fourth postoperative day, compared to 46% in the group managed with CPAP). Antonaglia et al14 compared IPV to standard CPT in 40 patients with COPD receiving noninvasive ventilation (NIV) in an RCT. They found that patients who received twice-per-day IPV therapy had improved oxygenation and carbon dioxide excretion, as well as decreased ICU stay. However, sputum production was not measured during the study and the incidence of pneumonia was not different. Therefore, the reason for improved ICU stay was unclear.
IPV has also been reported to be an effective treatment for atelectasis that is refractory to traditional therapies. In a small randomized trial, Deakins and Chatburn15 found that pediatric patients who received IPV had improved atelectasis scores by chest radiograph within 3 days, compared to no improvement among those receiving a standard CPT regimen for 6 days. Likewise, Reper and van Looy16 described 10 non-intubated patients with smoke inhalation injury and respiratory distress in whom the addition of IPV to standard CPT resolved atelectasis and improved PaO2/FIO2. Of particular interest was the fact that bronchoscopy had failed to treat atelectasis effectively.
Tsuruta et al17 superimposed IPV with traditional mechanical ventilation to treat compression atelectasis prospectively in a series of 10 obese patients with various etiologies of acute respiratory failure. Improvement in atelectasis was confirmed by either chest radiograph or chest computed tomography. However, these improvements were in the context of management using modest levels of PEEP (mean of 8 cm H2O, range 5–12 cm H2O) for up to 12 h, so that the attribution of improvements to IPV is not completely clear. Clini et al18 studied whether adding twice-daily IPV to a standard CPT regimen would improve outcomes in patients requiring prolonged weaning in a randomized trial. Although the incidence of atelectasis and need for bronchoscopy was not different, the incidence of nosocomial pneumonia was significantly less in patients receiving supplemental IPV therapy.
Despite the theoretical advantages of IPV for enhanced secretion clearance, and the positive results of the aforementioned studies, another randomized trial comparing IPV to either conventional or lung-protective ventilation did not find significant differences in infectious complications, at least in patients with thermal injuries.19 However, secretion clearance was neither monitored nor an end point in these studies.
Interpreting the usefulness of IPV as an adjunct therapy to enhance secretion clearance during mechanical ventilation is problematic for a variety of reasons including:
Many studies were done in patients who did not require mechanical ventilation, or IPV was used as the primary mode of ventilation.
Relatively small sample sizes
Heterogeneity of patient populations studied (eg, cystic fibrosis, inhalation injury, COPD, neuromuscular disease)
The monitoring of different variables of interest and end points studied
These factors stymie attempts to evaluate the relative usefulness of interfacing IPV therapy during conventional mechanical ventilation. Furthermore, tracheobronchitis from poor humidification has long been a concern with IPV therapy, so that a heated humidifier should be used to condition the inspiratory gases. Despite these limitations, interfacing IPV with pressure-regulated modes may be worth considering in patients with inhalation injuries, copious thick secretions, and, perhaps, atelectasis refractory to moderate levels of PEEP. However, it should be kept clearly in mind that IPV has not yet been demonstrated to improve clinically meaningful outcomes.
Another aspect of IPV has been the relatively consistent findings of improved oxygenation in patients with ARDS.5,20–23 It may be a particularly effective means of improving oxygenation in patients with ARDS and traumatic brain injury, wherein the application of sufficient levels of PEEP needed to recruit the lungs might cause undesirable increases in intracranial pressure.5,21 This has been attributed largely to the effects of high-frequency oscillation in improving intra-pulmonary gas distribution through several mechanisms, including the effects of turbulent flow on longitudinal gas dispersion (the Taylor effect), pendelluft motion, asymmetrical gas velocity profiles within the airways, and cardiac mixing, as well as the traditional mechanisms of bulk gas flow and diffusion in the alveolar zone.24
However, it must be recognized that these results emanate from small, uncontrolled, retrospective studies, wherein there is a lack of systematic application of standard therapies such as PEEP, clearly defined criteria used to decide whether or not conventional therapies had indeed failed, and systematic, protocol-driven application of IPV. For example, in the study by Velmahos et al22 the improvement in PaO2/FIO2 with IPV coincided with a significant increase in mean airway pressure, compared to conventional ventilation with pressure and volume control modes (35–63%, respectively). A salient but nonsignificant trend toward increased PEEP and mean airway pressure also was reported by Salim et al.21
Although only low level evidence supports the use of IPV to improve oxygenation in patients with ARDS, the concept of superimposing a percussive ventilation pattern during traditional pressure-regulated ventilation is intriguing, as it may utilize alternative methods of improving intrapulmonary gas mixing that may allow for improved oxygenation at lower levels of traditional support (eg, tidal volume, rate, and PEEP requirements). This potentially may be useful in the maintenance of lung-protective ventilation goals in ARDS. However, this remains only conjecture that would require affirmation from an appropriately sized prospective randomized controlled study before it could be advocated as standard practice.
Mechanical Insufflation-Exsufflation
Mechanical insufflation-exsufflation is an artificial cough technique whereby the lungs are mechanically inflated with positive pressure, followed by the sudden application of negative pressure to the airway. Although the technique has been used since the 1950s,25 it was only in the early 1990s that it became widely used when the Cough Assist In-Exsufflator (Phillips/Respironics, Murrysville, Pennsylvania.) became commercially available. Mechanical cough-assist devices share mechanisms of action similar to IPV, in that both create shear forces in the airway that help mobilize secretions. Typically, positive and negative pressure swings of 30–40 cm H2O are applied in repetitions of 3–5 maneuvers to mobilize secretions.26 This therapy has been used primarily to promote pulmonary hygiene in patients with neuromuscular diseases.27
Major reasons cited for extubation failure in patients who have successfully passed a spontaneous breathing trial are ineffective cough and/or the presence of copious secretions.28,29 Moreover, patients who fail a trial of extubation often require a prolonged ICU as well as hospital stay, and also are at greater risk for hospital mortality.30
NIV has been used to treat respiratory distress following extubation, but has produced inconsistent results.31 However, particular attention has not focused on the role of secretion clearance in preventing reintubation in these patients. Two recent studies32,33 investigated incorporating mechanical cough-assist devices with NIV to prevent extubation failure. In a small, prospective study,32 patients with neuromuscular disease recovering from acute respiratory failure were managed during the post-extubation period with NIV and mechanical cough-assist therapy. These patients were compared to historical controls who received standard medical therapy. Whereas all patients in the control group required reintubation, only 30% of patients in the prospective treatment cohort failed NIV and mechanical cough-assist therapy.
In another prospective study,33 75 patients who passed a spontaneous breathing trial were randomized to receive protocolized care involving NIV either with cough-assist therapy or with usual care during the first 48 h following extubation. NIV was initiated in either group only when predetermined signs of respiratory distress developed. In the experimental arm of the study, mechanical cough-assist therapy was initiated once prior to extubation, and continued with daily therapy sessions (3 treatments per day) for an additional 8 days. Significantly fewer patients in the mechanical cough-assist group required reintubation, compared to those who received NIV and usual care alone (17% vs 48%, respectively, P < .05).
Although these results are encouraging, there are several potential problems that could arise when introducing mechanical cough-assist therapy in a general ICU population. In patients at risk for sudden lung collapse (eg, ARDS, morbid obesity, abdominal compartment syndrome) disconnection from mechanical ventilation along with the application of high negative airway pressure could result in sudden profound hypoxemia. In such patients who also present with copious thick secretions, IPV therapy would appear to be a more reasonable and safer therapeutic option. As pointed out by others, mechanical cough-assist therapy cannot be recommended in non-intubated patients with severe respiratory distress and hypoxemia, or anxiety/agitation.34
Aerosolized Medications
Probably the most important adjunctive therapy during mechanical ventilation is the delivery of aerosolized pharmacologic agents. Historically, aerosolized drug delivery during invasive mechanical ventilation has been less efficient, compared to spontaneous breathing in the absence of an artificial airway. For example, only 2.9% of an administered dose via mechanical ventilation has been reported to actually reach the lower respiratory tract, compared to 11.9% when an artificial airway is not present.35 The paramount problem is substantial drug loss from impaction upon the artificial airway, caused by flow resistance.35 However, a host of other factors limit efficient aerosolized drug delivery, and these have been classified as ventilator-related, circuit-related, and device-related (Table 2).36–41 For the purpose of this discussion, both ventilator and circuit-related problems will be discussed within the context of aerosol delivery devices: pressurized metered-dose inhaler (pMDI) and nebulizer.
Factors That May Promote Better Pulmonary Aerosol Deposition
Traditionally, pMDI therapy has been reported to yield a higher deposition within the lower respiratory tract, compared to nebulizers (0.3–97.5% vs 0–42%, respectively), but these findings must be viewed within a historical context of different methodologies and lack of an accepted standardized model for aerosol testing.41 Under conditions of standardized testing, similar results have been found between delivery devices (ie, approximately 15%).40,41 In general, pMDI bronchodilator and corticosteroid therapy has been widely employed during mechanical ventilation, as it is considered to be more economical with less risk for nosocomial pneumonia, compared to nebulized therapy.41 However, with the conversion from generic chlorofluorocarbon to brand name hydrofluoroalkane propellants, the economic advantages of using pMDI may diminish.40 Furthermore, advances in nebulizer technology have lessened the problems around drug wastage, thus improving both its efficiency and cost. For example, it was recently reported that using newer nebulizer technology to deliver bronchodilators to mechanically ventilated patients may reduce costs by approximately 80%, compared to pMDI based therapy.38 Below is a review of the advantages and disadvantages of both pMDI and nebulizers, followed by a discussion of various non-bronchodilator, adjunctive aerosol therapies during mechanical ventilation.
Pressurized Metered-Dose Inhalers
When pMDIs are used, drug delivery is strongly influenced by the choice of pMDI adapter, the position of the adapter within the ventilator circuit, both use and type of reservoir chamber, the timing of pMDI actuation, whether inspired gases are conditioned with heat and humidity, and the type of pMDI used.36,40,41 For example, improved bronchodilator effects of β agonists occur when a reservoir chamber is placed 15 cm proximal to the circuit Y-piece-adapter.42 More subtle details, such as whether the reservoir chamber is unidirectional or bidirectional,43 matching the pMDI canister stem with the actuator,44 and simply assuring that the pMDI canister is warmed and shaken (to assure proper mixing of drug and propellant),36 also can affect the efficiency of drug delivery during mechanical ventilation.
Actively heating and humidifying inspired gases during mechanical ventilation reduces aerosol deposition by approximately 40%,41 and is believed to be caused by hygroscopic growth of aerosol particles that are filtered out by the artificial airway. Yet turning off or removing active humidification from the ventilator circuit during treatments is not recommended,36 as it is both impractical and introduces additional problems. These include allowing sufficient time for the ventilator circuit to cool and dry (thus increasing duration of therapy), frequent circuit disconnections that increase the risk of both lung de-recruitment and inadvertent circuit contamination, and damaging the airway mucosa from repeated exposure to dry gases. This last point is particularly important if humidification inadvertently is not resumed following therapy, as might occur during high work load periods in a busy ICU. Furthermore, reduced aerosol delivery occurs when a heated wire circuit is used, particularly if substantial condensation forms in the spacer and tubing.45 Interestingly, shutting off the humidifier for 10 min prior to therapy appears to have little impact on improving drug delivery.44 In contrast, passive humidification with a heat and moisture exchanger creates a formidable barrier to aerosol delivery and must be removed prior to therapy unless a specific bypass device is used.
Another potential barrier to pMDI efficiency is its use during spontaneous modes of ventilation. Asynchrony between pMDI actuation and the onset of inspiratory flow can substantially reduce drug delivery, particularly if actuation occurs during expiration.46 This issue becomes important when pMDIs are used during spontaneous modes of ventilation such as pressure support ventilation, as appropriate synchronization is difficult.
The breathing pattern during mechanical ventilation also impacts drug delivery. A sufficiently large tidal volume of approximately 500 mL (7–8 mL/kg for an average-sized adult), long inspiratory time and slow inspiratory flow (30–50 L/min) are required to optimize drug delivery.36,40,41 These variables cannot be controlled when spontaneous breathing modes are used, particularly in the presence of tachypnea. Although these factors also are in play when nebulizers are used, the fact that drug delivery is based upon only a very limited number of actuations per treatment (compared to 5–10 min of sustained therapy with a nebulizer) may render the use of pMDI more vulnerable to inefficiency.
Nebulizers
When nebulizers are used, important considerations include the position of the nebulizer and use of a reservoir chamber, the type of nebulizer used, fill volume, breathing frequency, inspiratory time, inspiratory flow, inspired tidal volume, triggering mechanism (ie, pressure vs bias flow triggering), whether inspired gases are conditioned with heat and humidity as well as type of humidifier used, the gas flow used to power nebulization, and whether nebulization occurs continuously throughout the respiratory cycle or is synchronized with inspiration.36,40,41
There are 3 distinct types of nebulizer commonly used (ie, jet, ultrasonic, and vibrating mesh or plate), each with unique features. Therefore, considering the pros and cons of nebulizer choice becomes more complicated. However, some of the same adjustments and limitations described for pMDIs apply equally for all nebulizers. For example, proximal placement of a nebulizer in relation to the circuit Y-piece-adapter,47 along with incorporation of an aerosol reservoir,48 increases drug delivery to the patient. Heated, humidified gases also have a negative impact, accounting for substantial reductions in drug delivery.49
In particular, when jet nebulizers are used, consideration must be given to the fact that aerosol production and particle size may vary not only between brands but also between different batches within the same brand.50 The gas flow source used to power a jet nebulizer introduces another level of complexity. Using a high-pressure external flow meter to power the jet nebulizer can produce a higher proportion of aerosol particles capable of deposition within the lower respiratory tract (ie, mass median aerodynamic diameter of 1–3 μm). However, each model of jet nebulizer is designed to work at a specific flow range, which is usually stated by the manufacturer. Typically this is 6–8 L/min.36,39,41,47 Powering a jet nebulizer at a flow rate below its recommended range renders the device less efficient, causing increased drug retention within the nebulizer and producing larger, more heterodispersed particles that tend to be filtered out by the artificial airway.47
A jet nebulizer powered by an external flow meter produces aerosol throughout the ventilatory cycle, thus increasing drug wastage. Likewise, the use of bias flow triggering, particularly at flows > 2 L/min, will increase drug wastage.36 Synchronized, intermittent nebulization using the internal nebulizer function on ventilators reduces drug wastage.49,51 Historically, this technique was commonly avoided because on many older ventilators the intermittent nebulization feature was substantially under-powered (eg, 15 psi).52 This resulted in excessive treatment duration and insufficient gas flow to generate respirable particle sizes. Also there is a tendency for preferential aerosolization of normal saline diluent with jet nebulizers that results in a residual or “dead volume” of 0.1–2.4 mL containing a higher drug concentration.36 Because jet nebulizers do not function below the dead volume, less drug is delivered to the patient. Increasing the fill volume lessens the amount of drug retained within the nebulizer.36,41,47
An ultrasonic nebulizer produces aerosol through vibration of a piezo-electric crystal. Its performance is related to the frequency and amplitude capabilities, so that the efficiency of any nebulizer may vary between manufacturers.41 An advantage of ultrasonic nebulizers is their higher rate of aerosol output and shorter duration of therapy.53,54 Although better deposition, compared to jet nebulizer, has been reported with newer models,53 the impression is that the ultrasonic nebulizer technology requires more development in order to achieve wider acceptance.55 For example, the versatility of ultrasonic nebulizers in clinical practice may be restricted due to heating issues. In contrast to jet nebulizers (in which drug solutions cool during aerosolization), ultrasonic nebulizers tend to heat up solutions as much as 20°C over 6 min.56 This could denature some drug preparations, rendering them ineffective. And it already appears that ultrasonic technology has been supplanted by newer technologies.40
The newest nebulization technique for clinical practice uses a vibrating mesh or plate to generate an aerosol output 2 to 3 times greater than jet nebulizer, with a nominal antibiotic dose delivery of 60%, with virtually no residual volume.40 A 2 to 4 fold higher albuterol delivery also has been reported with a vibrating mesh nebulizer, compared to jet nebulization, during mechanical ventilation.57 Another advantage of a vibrating mesh nebulizer is that it can aerosolize proteins and peptides without risk of denaturation and therefore opens more opportunities for inhaled pharmacotherapies.40
In summary, several practical steps can be taken to improve aerosolized medication delivery to the lungs with both pMDI and nebulizer delivery systems. Although pMDI has long been considered the more efficient and economic delivery method, improvements in nebulizer technology and the recent changeover to brand name hydrofluoroalkane propellants may diminish, if not remove, that rationale. In particular, the advent of vibrating mesh nebulizer technology appears to be better suited for delivering an increasingly diverse array of drugs.
Aerosolized Antibiotics
The reported incidence of VAP is 8–28%,58 and VAP is the most frequent hospital-acquired infection among surgical ICU patients.59 VAP is the leading cause of death among critically ill patients with hospital-acquired infection, and has an associated mortality between 25–50% (as high as 76% in some circumstances).58 Also, it accounts for more than 50% of antibiotic use in the ICU.60 In particular, VAP caused by Pseudomonas aeruginosa is very difficult to treat, as it is characterized by both recurrent infection and a high tendency toward antibiotic resistance, despite appropriate antibiotic management.61
Moreover, systemic antibiotics may not be ideal for treating pneumonia. Achieving an appropriately high concentration capable of eradicating bacterial reservoirs residing within thick secretions and biofilm is difficult. It requires drug dosages that may increase the likelihood of systemic toxicity,62 as well as eliminate the normal flora of the gastrointestinal tract. This, in consequence, paradoxically promotes the selection of multi-drug resistant (MDR) organisms.63 In our current situation, marked by an increasing prevalence of MDR pathogens, and coinciding with a shrinking array of effective antibiotics, there is renewed interest in using aerosolized antibiotics to treat both VAP and ventilator-associated tracheobronchitis (VAT). VAT is considered a precursor to VAP, and both conditions share similar microbiologic and features of presentation.64 However, the most salient distinctions are that while VAT also presents after 48 h of mechanical ventilation with both fever and leukocytosis (or leukopenia), worsening oxygenation and chest radiographic findings of a new, persistent or worsening infiltrate may be absent.
For several decades aerosolized antibiotics have been used successfully to treat both cystic fibrosis and Pneumocystis jiroveci pneumonia.65 In particular, the efficiency of vibrating mesh nebulizer technology may be an important step in advancing aerosolized antibiotic therapy.57 Several small RCTs, as well as uncontrolled studies,66–77 have been published (Table 3). Although not uniformly unambiguous, many of these studies suggest that aerosolized antibiotics maybe a more effective strategy to treat VAP and VAT.
Contemporary Studies of Aerosolized Antibiotics for the Treatment or Prevention of ventilator-Associated Pneumonia
Since 2000 there have been 6 small prospective RCTs that have examined aerosolized antibiotics for the treatment67,72,74,77 or prevention of VAP.75,76 Of the treatment trials, those done by Palmer et al67 and Lu et al72 merit further discussion. Palmer et al67 studied 43 patients with VAT, randomized to receive either aerosolized normal saline or aerosolized antibiotics, based upon the Gram stain of sputum (ie, 120 mg vancomycin every 8 h for Gram-positive bacterial infections, or 80 mg gentamicin every 8 h for Gram-negative bacterial infection) in addition to intravenous antibiotic therapy. Those treated with aerosolized antibiotics had a significant reduction in signs of respiratory infection, white blood cell count, bacterial resistance, and use of intravenous antibiotics, compared to the control group. Fewer patients in the treatment group required additional antibiotics, either for new or persistent infection, compared to the control group (42% vs 71%, respectively). Although 28-day mortality was not different between the groups, among survivors 80% of those receiving aerosolized antibiotics were successfully weaned during the study, compared to 45% in the control group (P = .046). Also there was a strong tendency toward higher ventilator-free days among those in the treatment group, compared to the control group, (median of 10 vs 0, respectively, P = .07).
Recently, the Nebulized Antibiotics Study Group72 published the results of a phase II RCT comparing an 8-day course of aerosolized versus intravenous administration of both ceftazidime and amikacin in 40 patients with confirmed VAP caused by Pseudomonas aeruginosa. By carefully measuring the amount of drug sequestered in the nebulizer and ventilator circuit, it was estimated that approximately 63% of the nebulized dose was delivered to the respiratory tract. Compared to intravenous therapy, those patients receiving aerosolized antibiotics had a tendency toward higher resolution of VAP (55% vs 70%, P = .33, respectively) and less treatment failure (30% vs 15%, respectively), as well as more rapid reduction in bacterial growth. The investigators did report a small number of important adverse events with aerosolized antibiotics, including a 25% decrease in PaO2/FIO2 after treatment in 3 patients (15%) and expiratory limb circuit filter obstruction in another 3 patients, one of whom suffered a cardiac arrest.
There have been 2 recent prophylaxis studies75,76 of aerosolized antibiotics in trauma patients. Critically ill patients with traumatic injuries seemingly are more susceptible to developing VAP than the general ICU population, and this tends to be associated with increased morbidity and mortality.78 In a preliminary safety/efficacy study, Wood et al75 found an impressive 73% reduction in the incidence of VAP among trauma patients treated for one week with aerosolized ceftazidime, compared to placebo. For the entire ICU stay the incidence of VAP was 54% lower. These positive results were associated with significant reductions in alveolar concentrations of pro-inflammatory mediators (eg, tumor necrosis factor alpha and interleukin 1-beta). In addition, the investigators delimited the study to those patients at high risk for VAP and restricted treatment exposure so as to reduce the chances of promoting the selection of MDR microorganisms. As a result, the bacterial flora and sensitivity patterns were not altered.
Unfortunately, in a larger follow-up study76 the same investigators were unable to duplicate their results, as the incidence of VAP (both at 2 weeks and 30 d) was the same. However, it was noteworthy that the number of patients who developed infections with MDR microorganisms was not different, thus supporting previous observations that brief courses of aerosolized antibiotics do not appear to alter the microbiologic ecology in the ICU setting.
Several retrospective studies also merit discussion. Among these, Michalopoulos et al68 reported on 60 patients who were treated prospectively with aerosolized colistin as an adjunct to intravenous antibiotic therapy for VAP. Fifty (83%) patients showed a positive response to therapy (eg, improved chest radiograph and arterial blood gases, normalization of white blood cell count, C-reactive protein, and procalcitonin levels) without adverse effects. Likewise, Mohr et al66 reported significant improvement in oxygenation (PaO2/FIO2 increased from 201 ± 92 mm Hg to 250 ± 90 mm Hg, P < .05), and no adverse pulmonary effects or renal toxicity from aerosolized tobramycin or amikacin among 22 patients with VAP. In terms of efficacy in treating infection, the results were mixed. Whereas 45% of patients had resolution of VAP and complete eradication of the responsible pathogen, pneumonia recurred in 41%, but in only one case did the responsible microorganism become newly MDR.
Ghannam et al69 also reported no adverse effects from either aerosolized tobramycin or colistin in a retrospective case-control study. Therapeutic efficacy in this study was impressive, as all patients treated with aerosolized antibiotics had complete resolution of pneumonia, compared to 55% of those receiving intravenous antibiotic therapy alone (P < .01). Also, among patients who had follow-up lower respiratory tract cultures, bacterial eradication was complete in 77% of patients who received aerosolized antibiotic therapy, compared to 8% among those receiving intravenous antibiotic therapy alone (P < .001). Most recently, in a retrospective study of patients with VAP, despite greater illness severity and infection with MDR strains of Pseudomonas aeruginosa or Acinetobacter baumannii, those who received either aerosolized tobramycin or colistin had significantly greater 30-day survival, compared to those who received intravenous antibiotic therapy alone.73
Despite these encouraging results from both prospective and retroprospective studies, aerosolized antibiotics cannot yet be recommended for general practice for several reasons. First is the need to determine clinical efficacy in a sufficiently sized multicenter phase III RCT. There are other concerns that suggest caution before advocating widespread use of aerosolized antibiotics. One is the non-uniform criteria that have been used to judge positive outcomes, such as whether improvement in clinical signs of infection is sufficient for defining efficacy (clinical resolution of infection), or whether the more arduous task of microbiological confirmation of pathogen eradication is necessary.59
Another is that 2 preliminary prophylaxis studies79,80 done in the 1970s showed a disturbing trend toward rapid selection for MDR as well as atypical microorganisms. In one study,79 selection of MDR microorganisms may have been related to the use of aerosolized antibiotics in low-risk patients who were not mechanically ventilated (ie, where antibiotic aerosol freely escaped into the environment). In the other study,80 acquired pneumonia was caused either by MDR or atypical pathogens and was associated with a higher than expected mortality rate (64%). The recent study by Claridge et al76 found that prophylactic treatment is not effective, thus suggesting the risk/benefit ratio of prophylactic, aerosolized antibiotic therapy is untenable. Again, it must be emphasized that the indiscriminant dispersion of antibiotic aerosol into the hospital environment during prolonged therapy (as might occur in non-intubated patients with traditional aerosol delivery systems) increases the likelihood that MDR and atypical microorganisms may emerge. However, restricting aerosolized antibiotic therapy to only intubated, mechanically ventilated patients, wherein antibiotic aerosol can be captured in the ventilator circuit, may greatly reduce this risk factor.
Given the unrelenting evolutionary pressure from emerging MDR microorganisms, a large, multicentered, double-blinded, RCT of aerosolized antibiotics for the treatment of VAP/VAT is of paramount importance to critical care practice. In the interim, however, it is reasonable to consider using aerosolized antibiotics in highly circumspect, individual cases of VAP; in particular, those cases caused by MDR microorganisms unresponsive to traditional intravenous antibiotic therapy,59 or when avoiding renal toxicity is of paramount importance.
Aerosolized Prostaglandins and Inhaled Nitric Oxide
Since the 1990s there has been interest in managing severe hypoxemia in ARDS with direct pulmonary delivery of vasodilators such as inhaled nitric oxide (INO),81 aerosolized prostacyclin (PGI2),82 and, more recently, alprostadil (PGE1).83 Because these inhaled drugs are distributed preferentially to ventilated areas of the lung, they cause selective pulmonary vasodilation and decrease hypoxemia by increasing ventilation/perfusion matching while avoiding the risk of systemic vasodilation.82 Although these therapies do not reverse the primary mechanism for hypoxemia (ie, alveolar flooding and collapse), they provide an alternative approach to supporting pulmonary gas exchange without increasing PEEP or FIO2. In patients with severe hypoxemia and hemodynamic instability, or in those with limited recruitable lung tissue, inhaled vasodilator therapy is attractive because it provides clinicians with more management flexibility. In addition, INO81 and aerosolized PGI284,85 reduce pulmonary vascular resistance and pulmonary arterial pressure. Furthermore, inhaled PGI2 has anti-inflammatory properties and inhibits platelet aggregation, which, in theory, may help ameliorate the progression of lung injury.86
Several RCTs have consistently shown modest to moderate short-term improvement in oxygenation in patients with ARDS treated with INO.87–90 The level of evidence supporting aerosolized PGI2 therapy in ARDS is much weaker, usually consisting of small uncontrolled studies85,91 and case reports92–94 describing modest to moderate improvements in oxygenation. However, this has not been found consistently among all investigations.83,95
It is important to stress that there is no evidence that inhaled vasodilator therapy improves outcomes in ARDS, and it may carry some risks for toxicity. Utilizing either INO or aerosolized PGI2 to treat refractory hypoxemia constitutes “off-label” use and is justified as a rescue therapy in cases of severe ARDS.86 High doses of INO can cause methemoglobinemia, and may cause renal dysfunction as well as direct pulmonary toxicity from the enhanced production of free nitrogen radicals that occur in a high FIO2 environment.96 However, these risks do not appear appreciable at currently recommended dose ranges of 5–20 ppm.86 Prolonged exposure to inhaled PGI2 has not been thoroughly studied in terms of toxicity. Nonetheless, short-term exposure at moderate doses (eg, 30 ng/kg/min) does not appear to produce pulmonary toxicity.96 Early, mild airway irritation has been reported in an animal model exposed to doses greatly exceeding the maximally recommended clinical dose of 50 ng/kg/min.97,98
In summary the use of both INO and aerosolized PGI2 therapy during mechanical ventilation should be restricted either to support pulmonary gas exchange in very severe cases of ARDS, or in the treatment of severe pulmonary hypertension. Currently, the evidence supporting improved oxygenation is stronger for INO than for aerosolized PGI2. Notwithstanding this imbalance of evidence, the higher costs associated with INO alone are justification for considering aerosolized PGI2 as an alternative. Moreover, it should be stressed that both therapies are strictly supportive and have not been shown to improve outcomes. These therapies may allow stabilization of gas exchange so that definitive therapies have a chance for success.
Aerosolized Anticoagulants and Oxygen Radical Scavengers
ARDS induced by smoke inhalation injury is strongly associated with mortality in patients with thermal injuries.99 Approximately 70% of patients with smoke inhalation injury develop acute respiratory failure,100 and this is a leading cause of death in burn victims.101,102 The major pathologic features of inhalational injury include fibrin deposition, cellular debris from epithelial sloughing, and increased mucus production leading to the development of airway casts and severe airway obstruction. In addition, the release of toxic radical oxygen species as a result of complement activation enhances endothelial injury and aggravates pulmonary edema. A combination of aerosolized heparin and N-acetylcysteine has been used in the management of inhalation injuries.103–105
Animal models of acute smoke inhalation lung injury have produced somewhat mixed results. In a sheep model of smoke inhalation injury along with sepsis induced by pulmonary instillation of Pseudomonas aeruginosa, subsequent treatment with heparin, either aerosolized (10,000 units) or intravenous (5,300 units/kg) reduced histologic changes, including pulmonary edema and cast formation.106 However, in a sheep model of smoke inhalation injury and extensive cutaneous burns, only a combination of aerosolized heparin and recombinant human antithrombin was found effective in improving pulmonary gas exchange and attenuating lung injury.107
To date, the best evidence in support of this therapy is from 2 retrospective, single-center, case-control studies of bronchoscopy-confirmed inhalation injury in both pediatric,103 and adult patients.104 When compared to controls, pediatric patients treated during the first 7 days of injury with a combination of aerosolized heparin (5,000 units) and 3 mL of 20% N-acetylcysteine every 4 h had significantly reduced atelectasis (69% vs 42%, respectively), reintubation rate, (28% vs 6%, respectively), and mortality (19% vs 4%). Similar findings of improved 28-day survival rate (94% vs 57%), as well as improved lung injury scores, and chest mechanics were reported in 28 adult patients.104 In general, aerosolized heparin therapy has not been associated with increased bleeding risks in patients with inhalation injury.103,105 However, a case of clinically important coagulopathy was reported in a pediatric patient with inhalation injury whose clinical course was complicated by disseminated intravascular coagulation.108
More recently, a preliminary study investigated the potential usefulness of aerosolized heparin for the management of ARDS from common etiologies such as pneumonia and sepsis.109 The rationale for utilizing aerosolized heparin in common causes of ARDS is the same as in smoke inhalation. Acute inflammation induces fibrin deposition, which causes both hyaline membrane formation in the alveolar space and pulmonary capillary microthrombosis. In the prospective, short-term (48 h), phase I study109 comparing 4 dose regimens (50,000–400,000 units/d), aerosolized heparin was found to be feasible in terms of drug delivery to the lung parenchyma and was not associated with serious adverse events. However, the potential efficacy of aerosolized heparin to improve pulmonary function was not addressed, and awaits the results of future phase II and phase III studies.
Heliox
Since the 1980s there has been a resurgence in using helium-oxygen gas mixtures to relieve respiratory distress in non-intubated patients with upper-airway obstruction from croup and post-extubation stridor,110 as well as from lower airway obstruction in status asthmaticus,111 COPD,112 and bronchiolitis.113 Most evidence supporting its use has come from low-level studies.114 Because heliox does not affect the underlying pathophysiology of airway obstruction, it is not surprising that its use in controlled studies has not improved outcomes.110 Nevertheless, it provides symptomatic relief and more flexibility in clinical management until the underlying conditions causing airway obstruction can be treated effectively.
Helium is biologically inert; it is essentially insoluble in tissues, and non-reactive with both cellular membranes as well as other common respiratory gases.113 The primary mechanism by which heliox works is the fact that helium is 86% less dense than air (0.179 vs 1.293 g/L, respectively), which reduces turbulence, thus decreasing airways resistance and work of breathing.115 Turbulence is characteristic of gas flow in the first 10 generations of human airways because of their branching architecture.112
Compared to air/O2 gas mixtures, helium/O2 mixtures have only marginally increased viscosity, but substantially lower density. This is referred to as “kinematic viscosity” or the viscosity/density ratio. Higher kinematic viscosity reduces the Reynolds number (that expresses the relationship of kinetic and viscous forces influencing gas flow) and converts areas of turbulent flow within the respiratory tract toward laminar flow (Fig. 3).111 In addition, the lower density of helium promotes CO2 elimination by altering the binary diffusion coefficient for intrapulmonary gas mixtures.116
Representation contrasting the effects of a restriction on gas flow based upon its “kinematic viscosity” (viscosity/density ratio). Restrictions or bifurcations in the airway produce turbulence or “eddies” that disrupt a smooth or “laminar” gas flow and create a high “back-pressure.” The degree of turbulence that develops is directly proportional to gas density; the lower the density, the less turbulence. In this illustration, changing the inspired gas mixture from a high oxygen concentration to one with a higher concentration of less-dense helium would tend to convert areas of turbulence in the larger airways toward more of a laminar flow pattern that should result in increased gas flow at a lower driving pressure.
One of the first reports on the incorporation of heliox during invasive mechanical ventilation was by Gluck et al,116 who described rapid and impressive reductions in both peak airway pressure (mean of 33 cm H2O) and PaCO2 (mean of 36 mm Hg) in 7 patients in status asthmaticus ventilated with a helium/oxygen mixture of 60/40%. Additional studies have reported similar results of reduced peak inspiratory pressures and improved gas exchange,117 as well as reduced intrinsic PEEP and work of breathing112 when heliox mixtures are used during mechanical ventilation.
In neonates with meconium aspiration, pressure control ventilation with heliox was associated with significant improvements in oxygenation, but only nonsignificant trends toward increased tidal volume and peak expiratory flow.118 In a pediatric case of extraordinarily severe ARDS, heliox was used in conjunction with high frequency jet ventilation, which resulted in a rapid improvement in ventilation, as PaCO2 decreased from 57 to 34 mm Hg.119 Heliox also increases aerosol deposition in the lung periphery and therefore may improve the effectiveness of bronchodilator therapy.115 This has been supported indirectly by studies reporting improved pulmonary function and lower dyspnea scores when bronchodilator therapy with jet nebulizers are powered by heliox.112
When clinically acceptable gas exchange can be achieved with a reasonable degree of lung-protection, the costs associated with heliox do not justify its routine use during mechanical ventilation for patients with obstructive lung disease unless there exists profound air-trapping in the presence of hypotension and severe acidosis. Heliox is a useful tool in the clinical armamentarium; however, it should be reserved for patients with severe acute respiratory failure from obstructive lung diseases.
Summary
The clinical evidence reviewed in this paper suggests that:
First, interfacing IPV with pressure-regulated modes of ventilation may benefit patients with either inhalation injuries or copious thick secretions, whereas artificial coughing with a mechanical insufflation-exsufflation device appears to be effective in patients with neuromuscular disease.
Second, of all inhaled drug therapies currently being investigated, probably none is more important than clearly determining whether aerosolized antibiotics should be used to treat VAP, particularly VAP caused by MDR pathogens resistant to traditional systemic treatment. However, the lack of a definitive phase III RCT, along with the potential risk of inadvertent promotion of MDR microorganisms, prevents advocating the general use of aerosolized antibiotic therapy for the treatment of VAP/VAT.
Third, INO or aerosolized PGI2 therapy should be restricted either to support pulmonary gas exchange in cases of severe ARDS or in the treatment of severe pulmonary hypertension. Although both therapies are strictly supportive and have not been shown to improve outcomes, they may “buy time” to allow definitive therapy to work.
Fourth, very low level clinical evidence suggests that inhaled anticoagulants and oxygen radical scavengers may promote bronchial hygiene and reduce inflammation in ARDS induced by smoke inhalation injury.
Finally, heliox is a useful tool in the clinical armamentarium. But because of its cost, heliox should be reserved for patients with severe acute respiratory failure from obstructive lung diseases.
In this era of evidence-based practice there is justifiable hesitancy toward advocating therapies lacking sufficiently strong clinical data to support their use. Nowhere does this seem more applicable than to the use of adjunctive therapies during mechanical ventilation. Most adjunctive therapies described here are incapable of reversing the underlying pathology under conditions of severe critical illness or trauma (eg, ARDS and overwhelming infection or injury). However, these therapies appear to be useful in supporting the patient until definitive treatment can be undertaken and given sufficient time to work. From a purely practical perspective, it also is apparent that limitations (both financial resources and perceived relative importance) will prevent the undertaking of large RCTs sufficient to provide definitive answers to many of the clinical issues highlighted here.
Therefore, in the absence of compelling evidence, how should clinicians approach the incorporation of adjunctive therapies during mechanical ventilation? A reasonable approach to unproven therapies, such as those described above, might be that they are: used with caution and circumspect enthusiasm; used conservatively with clearly established indications and guidelines or protocols for implementation as well as discontinuation; monitored carefully through ongoing quality assurance projects that routinely reassess the risk and cost-to-benefit ratio; and willingly abandoned if compelling evidence arises (either internally, or through peer-reviewed publications) strongly suggesting harm or ineffectiveness.
Footnotes
- Correspondence: Richard H Kallet MSc RRT FAARC, Respiratory Care Services, San Francisco General Hospital, NH GA-2, 1001 Potrero Avenue, San Francisco CA 94110. E-mail: rich.kallet{at}ucsf.edu.
Mr Kallet presented a version of this paper at the 51st Respiratory Care Journal Conference, “Adult Mechanical Ventilation in Acute Care: Issues and Controversies,” held September 7 and 8, 2012, in St Petersburg, Florida.
The author has disclosed no conflicts of interest.
- Copyright © 2013 by Daedalus Enterprises
References
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- 64.↵
- 65.↵
- 66.↵
- 67.↵
- 68.↵
- 69.↵
- 70.
- 71.
- 72.↵
- 73.↵
- 74.↵
- 75.↵
- 76.↵
- 77.↵
- 78.↵
- 79.↵
- 80.↵
- 81.↵
- 82.↵
- 83.↵
- 84.↵
- 85.↵
- 86.↵
- 87.↵
- 88.
- 89.
- 90.↵
- 91.↵
- 92.↵
- 93.
- 94.↵
- 95.↵
- 96.↵
- 97.↵
- 98.↵
- 99.↵
- 100.↵
- 101.↵
- 102.↵
- 103.↵
- 104.↵
- 105.↵
- 106.↵
- 107.↵
- 108.↵
- 109.↵
- 110.↵
- 111.↵
- 112.↵
- 113.↵
- 114.↵
- 115.↵
- 116.↵
- 117.↵
- 118.↵
- 119.↵
Discussion
MacIntyre:
In 1995 there was a consensus conference on innovations in mechanical ventilation,1 and one of the themes that came from that was that if you had an innovation that was not particularly risky or expensive, the required level of evidence for adopting it could be physiologic or intermediate types of outcomes. In contrast, an innovation with substantial risk or cost would not be recommended without support from a real outcomes study. That's my prologue to asking for your thoughts on INO, which is ridiculously expensive, has only transient benefits, and the RCTs to date have not shown any benefits or positive outcomes of any sort.2 The Canadian group3 even suggested there was a risk of renal failure. So if INO is both expensive and has risks, why do we even talk about it without the outcome evidence to support its use?
Kallet:
A good question. I agree with you; the ethics that you just laid out are clear. It's more of an emotional reaction, but when you're at the bedside in a dire situation and you think you're about to lose someone and you can't get the PO2 up, the cost/benefit ratio in that situation would, I think, allow the use of INO. If you're not at that point, I would have trouble with it. It gets my dander up when some people at prestigious institutions in the Bay Area (who shall remain nameless) say, “INO doesn't improve outcomes, so we don't use it: period!” I just can't buy into that.
MacIntyre:
Sounds pretty reasonable to me.
Kallet:
I know. But I think there are individual cases in all these questions of evidence-based medicine. It depends on the individual patient. I agree that INO should be restricted and not used as a general approach, but I think there are individual cases where it may be appropriate. That's my emotional response at the bedside, but I don't promote this.
MacIntyre:
I would call David Turner and get some help with ECMO [extracorporeal membrane oxygenation] in that kind of patient.
Turner:
We often use INO to help improve stability as a bridge to ECMO. There is often a transient improvement in gas exchange with INO, but if INO is not being used as a bridge to ECMO, an important question is when do you stop?
Kallet:
That's a good question, and I wish I knew. We've had the same problem with aerosolized prostacyclin. There's not much research on its pulmonary toxicity, as it is an alkalized solution that's inhaled for several weeks in some of our patients. So when should you begin to wean it down? I thought the same thing when I was touring a hospital that commonly uses ECMO in ARDS patients. There was a patient stabilized on 60% and PEEP of 10 cm H2O on ECMO, and I asked when do you start weaning off ECMO? They said “professional opinion.” It should be protocolized, and at our hospital we could do a much better job with this. That's the beauty of protocols: they kick clinicians in the butt and force them to move out of their comfort zone and challenge patients. As a culture we collectively don't do a very good job with that.
Kacmarek:
I don't think you gave prostacyclin its due in your presentation. You did mention the data on using it in pulmonary hypertension, but we're using it increasingly in the cardiac surgery arena. The cardiac surgeons, for the most part, find it equivalent to INO, and we have switched to 80% use of prostacyclin and decreased the use of INO in the cardiac arena. We also use prostacyclin for hypoxemic respiratory failure, but not as much as we use INO. Besides cardiac surgical patients, we see INO used in the pediatric and neonatal ICUs more than anywhere else for respiratory failure, but it tends to be a bridge to ECMO or a poor outcome.
Kallet:
You're absolutely right. I did shortchange the presentation on some things about aerosolized prostacyclin, particularly pulmonary hypertension, but my focus was mechanical ventilation.
Kacmarek:
I just didn't want cardiac surgeons to read this and get the wrong ideas.
Kallet:
We use it. We've seen some incredible cases; in one patient with extreme pulmonary hypertension we gave inhaled prostacyclin and within a few minutes there was a huge decrease in central venous pressure and increase in systemic blood pressure.
Marini:
I'd like to discuss secretions and ARDS, because I believe we are missing a lot by not paying attention to secretions. Secretions in the smaller airways can plug a lot of channels that would otherwise be open for gas exchange. When you keep a patient motionless, especially in the first few days, secretions are going to collect in the dependent areas. I wonder if it isn't high time for us to be looking at secretion management in patients with ARDS: particularly in primary ARDS, but also in edematous ARDS. The aerosolized heparin, digestive enzymes, et cetera that you talked about may also have a bigger role than we've been allowing them. We may be neglecting important therapies.
Kallet:
I agree. Hubmayr did some work looking for recruitment and basically found it was being sloshed back and forth.4 It hasn't received enough research emphasis. My own personal experience in some of the devastating hypoxemic cases has usually been severe ARDS complicated by lobar collapse from obstruction. It hasn't been focused on enough, and should be in future research. If the ARDS Network goes into a third iteration, it might be a good prospective RCT for them.
Schmidt:
One problem is systemic absorption. We observed substantial blood pressure drops with prostacyclin and with aerosolized antibiotics. With inhaled tobramycin we measured very high tobramycin levels in the blood. So the question is, does it work in the lung, or does it work systemically because we have another way of absorption? I'm not so sure about this; I'm a little bit skeptical.
Kallet:
One of the issues with prostacyclin is rain-out of aerosol where it can get into poorly ventilated areas and increase ventilation/perfusion mismatch after a while.
Schmidt:
Or blood pressure, because it gets systemically absorbed.
Kacmarek:
I think it's also related to dosing. We had problems in our earlier use, when we didn't have the dosing figured out to the extent we have now. We are not seeing the systemic hypotension, particularly in cardiac surgery patients, that we saw earlier. We adjusted the way we administer the drug and the dosage, and we found the right mix between benefit and avoiding adverse effects.
Schmidt:
True: much less.
Kallet:
It's hard with blood pressure, because we haven't seen the hypotension with long-term inhaled prostacyclin. Granted, it wasn't something we were necessarily looking for, but it wasn't obvious. Since we have a love affair with propofol and everybody's on high doses of propofol, it's harder in the ICU to know what's actually going on. I would think it would be a sustained problem, if aerosolized prostacyclin was being continuously systemically absorbed, we'd get some signal with blood pressure management. It's not something that's been very salient. It may be an issue, I don't disagree with you, Uli, but it's not something that's jumped out at us. And there are so many things going on in the critical care environment it's hard to figure out cause and effect.
Hess:
What about tachyphylaxis? One of our pharmacists advocates for increasing the dose—we use epoprostenol—when we have someone on for several days, because you develop some tachyphylaxis and you need to increase the dose.
Kallet:
Good point. We haven't looked at that. What I'd say clinically when we have someone on high doses of aerosolized prostacyclin is that we should be doing recruitment or proning. We should do something mechanically to recruit the lung and improve ventilation/perfusion to get them off that drug. It should be something to tide us over, get us over the hump, but I don't think we should rely on it for days at a time. People don't want to prone patients because it's a hassle, and they don't want to do recruitment maneuvers, which they think are dangerous. The drug doesn't appear to be dangerous and the perception is self-perpetuating. The ICU hums along as it always does. Clinicians a lot of times won't challenge themselves and get out of their comfort zone.
Branson:
I have a question about IPV. IPV makes perfect physiologic sense to me: it gives you a deep breath and then it creates the mucus flow. What's your feeling about our ability to monitor the actual volumes and airway pressures during IPV?
Kallet:
I don't know how you would track that, and I haven't really used IPV. We just started to use it with our burn patients. Do you have any suggestions for monitoring that?
Branson:
Dellamonica et al had a paper about the introduction of the IPV device inside the ventilator circuit.5 While most of the IPV aficionados will tell you that the phasitron [sliding venturi device that creates the IPV gas pulses] will prevent overpressurization because eventually it will get to where it doesn't entrain, if you set the driving pressure high enough, you can get quite a large tidal volume. That's something to be aware of.
Kallet:
Good point.
Blakeman:
A few years ago the Centers for Disease Control and Prevention was interested in the effect of aerosolized antibiotics on caregivers. On the walls of patient rooms they found substantial amounts of antibiotics. Can you speak to that?
Kallet:
I was focused on mechanical ventilation studies, and I didn't see that as an issue under those circumstances. The first thing that comes to mind is the administration of TOBI [inhaled tobramycin] in kids with cystic fibrosis, who aren't intubated. When we administered aerosolized pentamidine back in the 1990s, we had a filter on the expiratory circuit.
Blakeman:
When we use it in mechanical ventilation, we use TOBI and some colistin, and you can smell the antibiotics, even though we're filtering the ventilator circuit. There is some question of caregivers breathing it in and whether we might create super-bugs.
Kallet:
Yes, so wearing an N95 mask during aerosolization—I think in any RCT done with that, it should be an issue: clinician and environmental exposure need to be rigorously tracked. We need to convince people that the benefits outweigh the risks.
Gajic:
For cost reasons, in our institution we limited the use of INO for severe hypoxemia or shock in patients with pulmonary hypertension. However, we have a big group of adult congenital heart disease patients who come in with a new sepsis or something like that, and these patients may benefit from INO. We sometimes continue INO after extubation to NIV, to attempt to prevent reintubation, and some patients may pull through. Can inhaled prostacyclin work in that group of patients with severe pulmonary hypertension? Can it be used instead of INO?
Kallet:
I think they're using alprostadil.
Gajic:
No, you extubate and then you wean it off noninvasively.
MacIntyre:
They don't go home with INO?
Gajic:
No, but they live in the 80s. The point is that it's a new challenge. Before we restricted INO use for hypoxemic respiratory failure. We say, “If someone really wants to use INO, use inhaled prostacyclin. Neither works, but inhaled prostacyclin is cheaper.” For severe pulmonary hypertension, can INO be replaced by inhaled prostacyclin?
Kallet:
They're using inhaled alprostadil. Every 8 hours you give a treatment.
Hess:
Except it only lasts 2 or 3 hours.
Gajic:
In an acute situation can inhaled alprostadil replace INO?
Kallet:
I don't see why not.
Gajic:
It works as well?
Turner:
Yes, potentially.
Gajic:
Do you use it for babies?
Turner:
I do not have any personal experience with inhaled prostadil instead of INO for acute pulmonary hypertension, but some people are interested in it.
Gajic:
When visiting community hospitals, I still see some use of high-frequency jet percussive ventilation for patients with ARDS and secretions. Does anybody here use it, or what do you think about it?
Kallet:
We don't use it. We use run-of-the-mill lung-protective ventilation.
Turner:
We don't routinely use high-frequency percussive ventilation either, but there have been successful reports of its use, especially in burn patients.6,7
Gajic:
And does it work there?
Turner:
Some burn centers use it almost exclusively. There are certainly potential benefits in situations where secretion clearance is a major concern.
Branson:
The Army's burn group has used VDV [volumetric diffusive ventilation] for a long time. The original work on that was by Bill Cioffi. The Army burn team uses nothing but VDV for burned soldiers.8 Which is not to say that it works any better than anything else; it's what they do and it's their history. Things we've talked about for trying to improve secretions, both VDV and IPV, make perfect sense. The big concern with VDV has always been, like jet ventilation, the humidification. You humidify only the entrained gas, not the gas that's going in the end of the phasitron, which is the majority of gas delivered to the patient, so you can deliver some low humidity unless you're very careful.