Abstract
During the last few decades, attention has increasingly focused on noninvasive ventilation (NIV) in the treatment of chronic respiratory failure. The University of Leuven and the University Hospitals Leuven therefore chose this topic for a 2-day working group session during their International Symposium on Sleep-Disordered Breathing. Numerous European experts took part in this session and discussed (1) NIV in amyotrophic lateral sclerosis (when to start NIV, NIV and sleep, secretion management, and what to do when NIV fails), (2) recent insights in NIV and COPD (high-intensity NIV, NIV in addition to exercise training, and NIV during exercise training), (3) monitoring of NIV (monitoring devices, built-in ventilator software, leaks, and asynchronies) and identifying events during NIV; and (4) recent and future developments in NIV (target-volume NIV, electromyography-triggered NIV, and autoregulating algorithms).
- noninvasive ventilation
- amyotrophic lateral sclerosis
- chronic obstructive pulmonary disease
- sleep
- exercise training
- sleep monitoring
Introduction
Over the last 2 decades, long-term noninvasive ventilation (NIV) delivered by a nasal or oronasal mask has been well established in the treatment of patients with chronic hypercapnic respiratory failure arising from different etiologies.1,2 COPD, restrictive thoracic diseases, obesity-hypoventilation syndrome, and neuromuscular disorders are the main indications for long-term NIV.1–4 More recently, adaptive servo-ventilation (ASV) devices were developed to treat periodic breathing due to heart failure or other conditions associated with central sleep apnea.
Therefore, in February 2014, the University of Leuven and the University Hospitals Leuven organized a 2-day working group session on NIV to which numerous European experts in the field of NIV were invited. The aim was to review recent studies on NIV and to share insights and future directions on 4 major topics: amyotrophic lateral sclerosis (ALS), COPD, interpretation of NIV signals, and recent and future developments in NIV.
ALS
When to Start NIV?
Respiratory failure typically develops in the late stages of ALS, being the presenting feature in only 3% of patients.5 Respiratory symptoms include progressive dyspnea, orthopnea, nightmares, fragmented sleep, morning headaches, daytime sleepiness, and cough inefficacy. Inspiratory and expiratory muscle weakness can be assessed by routine respiratory function measurements.6,7 Patients with ALS usually die from hypoventilation,8 with hypoxemia and hypercapnia often precipitated by respiratory infections, aspiration pneumonia, or bronchial impaction.9
The first study of NIV in ALS reported that continuous daily use delayed or even eliminated the need for a tracheostomy.10 Two years later, Pinto et al11 published a prospective controlled trial with ALS subjects with respiratory failure and demonstrated that total survival and survival from respiratory symptom onset were better in subjects with ALS receiving NIV compared with palliative care. Two other studies described an increased survival with NIV use of > 4 h/d as opposed to those using less or refusing NIV.12,13
In 2006, Bourke et al14 published the only prospective randomized controlled trial (RCT) of NIV in ALS. Subjects began on NIV when presenting with either orthopnea and a predicted maximum inspiratory pressure of < 60% or symptomatic hypercapnia. In addition to increasing survival, NIV also improved quality of life (QOL), probably due to reduced nocturnal hypoventilation symptoms.13–15 Subanalysis suggested that survival and QOL significantly improved only in ALS subjects without bulbar involvement. In subjects with marked bulbar involvement, most authors report lower tolerance to NIV,13–16 possibly related to orofacial paresis, sialorrhea, and inefficient cough. Technical improvements and sialorrhea treatment might improve tolerance.
The timing to initiate NIV in patients with ALS is crucial due to the risks of rapid onset of respiratory failure, sudden death, and unanticipated invasive mechanical ventilation.16 The need for ventilatory support must be discussed with the patients and their families. The patient's decision must be respected.17 Mechanisms of action of NIV on symptoms and gas exchange may include: resting of respiratory muscles, resetting central CO2 receptors, improving respiratory mechanics, increasing pulmonary compliance, and resolving atelectatic segments. Over the last 15 years, 2 reports suggested that early NIV treatment could have additional benefits.18,19 Carratù et al20 found no significant difference in the 1-y survival rate between subjects not receiving NIV with an FVC of > 75% of predicted and those receiving NIV for > 4 h/d with an FVC of < 75% of predicted.
The first recommendations to initiate NIV were made in 1999 by the American Academy of Neurology (AAN) and the American College of Cardiology.3,21 In 2009, AAN criteria were updated.22 NIV was recommended in the presence of nocturnal hypoventilation symptoms (orthopnea, frequent awakenings, morning headaches, excessive daytime sleepiness, and inefficient sleep) plus one of: an FVC of < 50% of predicted, a maximum inspiratory pressure of < 60 cm H2O, a sniff nasal inspiratory pressure of < 40 cm H2O, or abnormal nocturnal oximetry. Recent European guidelines recommend NIV adaptation in ALS patients with one or more symptoms of respiratory failure (dyspnea, tachypnea, orthopnea, hypoxemia, sleep disturbances, morning headaches, respiratory accessory muscle usage, paradoxical respiration, daily fatigue, or sleepiness) and one or more objective parameters similar to the AAN criteria, except for an FVC of < 80% of predicted and the presence of hypercapnia.17
There is a general agreement that NIV must be initiated in the presence of respiratory symptoms in patients with ALS. However, there are still no clear recommendations about when to start NIV in asymptomatic ALS patients with abnormal respiratory tests. The decision must consider patients' will and comfort, QOL and survival benefits, health-care and caregiver support, and NIV costs.
NIV and Sleep
Sleep is often disturbed in patients with ALS.23–25 Lo Coco et al26 found poor sleep quality in 59% of subjects with ALS before the occurrence of hypoventilation. In the presence of diaphragmatic dysfunction, rapid-eye-movement sleep decreased, possibly as a defense mechanism against alveolar hypoventilation.
Few studies have evaluated the effect of NIV on sleep quality in subjects with ALS, and most of these studies were based on subject-reported outcomes.14,15,27–29 NIV improved sleep quality shortly after initiation27,29 and up to 10 months27 or longer,15,29 whereas daytime sleepiness decreased.15,28 Subjects with bulbar involvement seemed to be less tolerant of NIV, but subjects who tolerated NIV, even those with severe bulbar involvement, seemed to achieve better sleep quality.14
Katzberg et al30 used home polysomnography (PSG) before NIV and during follow-up. NIV was titrated during the daytime to ensure adequate mask fitting, volume, pressure, and breathing frequency targets. The only improvements were an increase in nadir SpO2 and the time spent overnight with oxygen levels below 90%. No improvement was found in sleep efficiency, arousal index, or sleep architecture. The authors suggested that a more meticulous NIV titration, by guidance with an additional PSG during NIV initiation, could have been helpful to optimize treatment.30 The choice of interface, ventilator settings, and the presence of bulbar involvement could possibly influence the effect of NIV on sleep. Atkeson et al31 found a high frequency of patient-ventilator asynchrony with an index of 69 ± 46/h of sleep and 17% of the nocturnal recording time spent in asynchrony. Once more, NIV was set up at home according to awake efficacy. A recent study in which NIV was titrated by in-hospital PSG with incorporation of transcutaneous CO2 measurement showed an improved sleep efficiency, sleep architecture, and arousal-awakening index in subjects without or with mild bulbar involvement.32
The effects of leaks and bulbar involvement on sleep quality and patient-ventilator asynchrony should be examined in future studies. Finally, the impact of reduced patient-ventilator asynchrony and enhanced sleep quality on clinical outcomes, including survival, should be evaluated.
Cough Augmentation
A recent Italian survey reported that difficulty in clearing secretions is one of the main reasons to refer subjects with ALS to a respiratory specialist.33 The ability to clear bronchopulmonary secretions is essential to prevent sputum retention and associated complications, including lower respiratory tract infection, which is the most common cause of hospital admission in these subjects.34 The act of coughing involves 3 main components: a deep inspiration up to 85–90% of total lung capacity, glottic closure for ∼0.2 s (which requires intact bulbar function), and effective contraction of the expiratory muscles to generate intrapleural pressures of > 190 cm H2O.35 If one or more of these 3 main components are impaired, coughing becomes less effective.36 Cough can be assessed simply and noninvasively in this group of patients. The inspiratory and expiratory components of the cough indicate the inspiratory and expiratory muscle function. Furthermore, asking patients to repeat the letter e helps in assessing their bulbar function. If the phonation of the letter e is normal, the bulbar function is intact.37 The simplest way to measure cough strength is to ask the patient to perform a cough peak flow through a face mask attached to a flow meter. A minimum assisted cough peak flow of 160 L/min is required to clear airway secretions.34,38,39 Patients with ALS should be taught cough augmentation techniques when their unassisted cough peak flow falls below 270 L/min, aiming to prevent secretion retention,38 respiratory infection, and subsequent respiratory decompensation.40 The consequence of a respiratory infection in these patients is a further reduction in respiratory strength and likely deterioration to the critical cough peak flow threshold of 160 L/min.34,38,39 In patients with ALS, a cough peak flow of >330 L/min indicates a survival of > 18 months.41
Assisted cough techniques should be targeted to the component of cough that is reduced. If sole expiratory muscle weakness occurs, a manually assisted cough will improve cough peak flow.42,43 In the presence of inspiratory weakness, the inspiratory component can be supported with maximum insufflation capacity via a face mask attached to a one-way valve and Ambu bag.44 If inspiratory and expiratory muscle weakness occurs, the inspiratory component can be supported with maximum insufflation capacity, and the expiratory component can be supported with a manually assisted cough.43 If the cough is extremely impaired (< 160 L/min), the patient may require mechanical insufflation-exsufflation. However, when bulbar function is severely impaired and maximum insufflation capacity is equal to vital capacity, the chances of improving cough strength are poor.45 It may be appropriate in these circumstances to consider a tracheostomy.
One study evaluating cough efficacy in ALS showed that greater improvements in cough peak flow were seen in non-bulbar compared with bulbar ALS subjects for both manually assisted cough and mechanical in-exsufflation.46 Sancho et al47 reported that stable ALS subjects with a cough peak flow of ∼245 L/min were able to clear secretions effectively when unwell with a manually assisted cough. However, those with a lower cough peak flow (155 L/min) required mechanical in-exsufflation for effective airway clearance. Vitacca et al48 reduced hospital admission in subjects with ALS by implementing a protocolized management approach. This included rapid access to home mechanical in-exsufflation devices if subjects were unable to clear secretions and improve SpO2 to > 95% on room air with other cough augmentation techniques.
In conclusion, subjects with ALS are at high risk of secretion retention. Routine measurements of cough peak flow should be carried out at each clinic visit. When cough peak flow is < 270 L/min, cough augmentation techniques should be taught. When cough peak flow is ∼245 L/min, a manually assisted cough may be sufficient to clear airway secretions. When cough peak flow is low (< 160 L/min), mechanical in-exsufflation devices are required. However, in patients with severe bulbar impairment, all cough augmentation techniques will possibly be ineffective. Access to mechanical in-exsufflation devices is recommended when managing patients with ALS.
Invasive Ventilation
In patients with ALS, NIV and mechanically assisted coughing may prolong survival, reduce hospitalizations, and improve QOL.14,49 In patients with severe bulbar involvement, these therapies are inadequate, and long-term invasive ventilation may be the only option to enhance survival. However, in most patients (30–92%), long-term invasive ventilation is started following endotracheal intubation due to a respiratory emergency.50–52 Although continuous NIV and mechanically assisted coughing may enable weaning from endotracheal intubation, most patients with saliva aspiration and glottic spasticity cannot be extubated.53
Following elective or emergency tracheostomy, median survival varied from 8 to 49 months (range of 0–155 months) and was significantly shorter in subjects > 60 y of age.50–52,54–56 The reported overall 1-, 2-, 3-, and 5-y survival was 37–78, 45, 23, and 12%, respectively. Survival of subjects receiving NIV followed by long-term invasive ventilation was much better compared with long-term invasive ventilation alone.
Very few studies have examined QOL in subjects with ALS following long-term invasive ventilation. Although these subjects frequently experienced depression, hopelessness, a feeling of loneliness, and loss of control, 85% estimated their QOL as acceptable with a positive attitude toward long-term invasive ventilation.54,57 In a questionnaire-based study, health-related quality of life (HRQOL) scores did not differ significantly between subjects receiving NIV and those receiving long-term invasive ventilation: 94% of the former and 81% of the latter would have chosen their mode of ventilation again. Although 97% of NIV caregivers would advise NIV, only 75% of long-term invasive ventilation caregivers would do so. Notably, 30% of long-term invasive ventilation caregivers rated their own QOL lower than that of the subject.58
Although the patient's interests are central, the impact on the caregivers (often family members) must be taken into account. Long-term invasive ventilation restricts the patient to total dependence on others and often imposes a very high burden on the caregivers. The most important factors reported in the decision-making process are QOL, severity of the disability, ability to return home, ability to discontinue long-term invasive ventilation, and concern about the family's QOL.59 Whether to propose long-term invasive ventilation remains a matter of debate, and there are marked differences in practice across Europe. In Italy, only 10% of pulmonologists chose not to initiate long-term invasive ventilation for ALS, compared with the majority of respiratory physicians in France, Switzerland, and The Netherlands, who encouraged shared decision making and early advanced care planning and who tended to discourage long-term invasive ventilation for ALS.33,60 Despite all doubts, long-term invasive ventilation may be indicated in the following situations: non-acceptance, intolerance, or ineffectiveness of NIV; failure to transfer to NIV following invasive ventilation; ineffective noninvasive management of airway secretions; or severe bulbar dysfunction with recurrent aspiration.
If professional home care is reimbursed, patients with ALS on long-term invasive ventilation may be discharged home.60 As most of these patients will need constant care and supervision to provide a safe environment, staying at home may become very expensive, with annual costs of over $400,000.60 When insurance companies are not willing to pay (anymore) and patients do not have financial support from family, friends, and volunteers, patients are force to decide between continued living in a nursing home or withdrawal from long-term invasive ventilation and death.
In conclusion, if patients with ALS want to prolong survival, long-term invasive ventilation may become the only option. Long-term invasive ventilation may improve survival at the expense of a further loss of functional capabilities, prolonged suffering, and high costs and burden of care. Apart from selected cases, long-term invasive ventilation should be discouraged. Shared decision making and advanced care planning are encouraged as early as possible. Emergency tracheostomy should be avoided by close monitoring of the ventilatory status and starting NIV or mechanically assisted cough instead of intubation in the acute care setting. There is a definite need for more studies into the merits and burdens of long-term invasive ventilation for ALS.
NIV and COPD: Recent Insights
High-Intensity NIV in COPD
Although the role of long-term NIV in the care of restrictive patients is undisputed, it is still a matter of debate as to whether long-term NIV should be used for patients with chronic hypercapnic respiratory failure arising from COPD.1–4 As a consequence, COPD was targeted for discussions on the best ventilatory strategies to use, focusing particularly on appropriate inspiratory pressures and backup frequencies.
In 2003, a meta-analysis concluded that 3 months of NIV did not improve lung function, gas exchange, or sleep efficiency in subjects with stable hypercapnic COPD.61 Four RCTs qualified to be included in this meta-analysis.63–66 Overall, PaCO2 non-significantly decreased by 1.5 mm Hg.62 Therefore, long-term NIV did not measurably augment alveolar ventilation. However, assisted (but not controlled) ventilation was used, and inspiratory positive airway pressures (IPAPs) were considerably low, ranging between 10 and 18 cm H2O.
Subsequently, 2 RCTs also assessed long-term outcomes in subjects with COPD receiving NIV.67,68 Again, ventilator settings were comparably low. As a consequence, physiological parameters (most importantly, PaCO2) did not improve. In addition, survival did not improve in one trial,67 whereas small survival improvements were reported at a cost of reduced HRQOL in the other trial following long-term NIV.68 Interestingly, the most recent meta-analysis concluded that insufficient evidence is available to support the use of routine NIV in subjects with stable COPD, but that higher IPAPs, better adherence, and higher baseline PaCO2 seem to impact improvements in physiology.69 Obviously, ventilator settings play a predominant role when deciding whether NIV is beneficial in stable hypercapnic COPD.
In an attempt to maximally decrease severely elevated PaCO2, pressure control ventilation was used with stepwise titration of mean IPAP up to 30 cm H2O in subjects with chronic hypercapnic respiratory failure due to COPD.70 This technique has been described as high-intensity NIV.71,72 Both physiological and clinical studies have further shown that improvements in blood gases, lung function, and breathing pattern are achievable with high-intensity NIV.73,74
Another important issue for patients on long-term NIV is HRQOL. The Severe Respiratory Insufficiency Questionnaire was designed to specifically assess HRQOL in patients receiving NIV.75–77 A multi-center trial confirmed that HRQOL benefits, measured by the Severe Respiratory Insufficiency Questionnaire, are substantial in patients with COPD when NIV is instituted, and overall HRQOL benefits were comparable to those for subjects with restrictive diseases.78 Finally, an RCT using a crossover design demonstrated that subjects with COPD who were familiar with controlled NIV and high IPAPs (29 ± 6 cm H2O) were also able to use NIV while walking by placing the device on a rollator. In this setting, oxygenation, dyspnea, and walking distance were substantially improved when NIV was added to supplemental oxygen.79
One study on subjects with stable hypercapnic COPD directly compared the new concept of high-intensity NIV (mean IPAP 29 cm H2O) with the conventional approach using considerably lower IPAP (mean IPAP 15 cm H2O), which has been termed low-intensity NIV.80 In this randomized crossover trial, the mean treatment effect between low- and high-intensity NIV, both used for 6 weeks at home, was > 9 mm Hg for nocturnal PaCO2, which served as the primary outcome, in favor of high-intensity NIV. Therefore, high-intensity NIV was shown to be superior to the conventional and widely used low-intensity NIV in terms of controlling nocturnal hypoventilation. As a consequence, the novel approach of high-intensity (but not low-intensity) NIV improved dyspnea during physical activity, lung function, and HRQOL as specifically measured by the Severe Respiratory Insufficiency Questionnaire.
One might speculate that high-intensity NIV with controlled ventilation and high IPAPs would not be nearly as well tolerated as low-intensity NIV with assisted ventilation and almost 50% lower IPAPs. However, one study revealed the opposite to be true, as subjects spent an average of an additional 3.6 h/d on high-intensity NIV compared with low-intensity NIV.80 In addition, dropouts occurred only while on low-intensity (but high-intensity) NIV. Thus, more effective ventilation achieved by more aggressive forms of NIV resulted in better subject adherence, which could be attributed to improved HRQOL and better symptom control. However, it should be mentioned that more days (2.5 d on average) were spent in the hospital to acclimatize subjects to high-intensity NIV compared with low-intensity NIV. This seems to be justified given the clear advantages of high-intensity NIV. For this reason, high-intensity NIV offers a new and promising therapeutic option in the treatment of COPD patients with chronic hypercapnic respiratory failure.
Despite these benefits, it has been argued that high-intensity NIV may substantially increase air leaks and therefore lead to sleep disturbances.80 These concerns were addressed by Dreher et al,81 who comparably measured sleep quality, as assessed by PSG, during low- and high-intensity NIV using a crossover approach. Again, high-intensity NIV was reportedly superior to low-intensity NIV regarding gas exchange, as already shown by previous trials.70–72,80 Importantly, sleep quality was comparably good during both approaches, with high-intensity NIV clearly not producing impaired sleep quality. Thus, this is further evidence to support the use of high-intensity NIV in patients with stable hypercapnic COPD.
The most recent RCT demonstrated both substantial survival and HRQOL benefits gained by long-term NIV established electively in subjects with COPD.82 In this study, subjects were recruited from 36 respiratory units in Germany and Austria. This was a large study, with 195 subjects being randomly assigned to the NIV group (n = 102) or the control group (n = 93). The 1-y mortality was 12% (12 of 102 subjects) in the NIV group and 33% (31 of 93 subjects) in the control group, with a hazard ratio of 0.24 (95% CI 0.11–0.49, P < .001). The mean inspiratory pressure was 22 cm H2O. The mean backup frequency was 16 breaths/min (range of 2–24 breaths/min), and 69% of the subjects had backup frequency of 14 breaths/min or higher. However, it must be noted that data on ventilatory pressures and backup frequencies were available for only 83% of the subjects receiving NIV. Nevertheless, long-term NIV was sufficient to lower elevated PaCO2 and to maintain the reduced PaCO2, with the 1-y change from baseline PaCO2 reported to be 7.4% (95% CI −8.6 to −6.2%). There were also significant changes in HRQOL as assessed by the 36-item Short Form questionnaire, St George Respiratory Questionnaire, and Severe Respiratory Insufficiency Questionnaire. This study clearly contrasts with all previous long-term trials, which may be due to the NIV settings, which were aimed at substantially reducing elevated PaCO2, as has been proposed for high-intensity NIV. In conclusion, there are now robust data to support that long-term NIV has the potential to improve HRQOL and long-term survival in subjects with stable hypercapnic COPD, but physiologically efficient ventilatory strategies capable of improving hypercapnia are mandatory for NIV success.82
Nocturnal Ventilatory Support in Addition to Exercise Training
Pulmonary rehabilitation was defined by the 2013 American Thoracic Society/European Respiratory Society statement and 2013 British Thoracic Society guidelines as an interdisciplinary comprehensive intervention of care for patients with chronic respiratory impairment that includes, but is not limited to, exercise training, education, and behavior change. The intervention is designed to optimize the physical and psychosocial performance and autonomy of the patient.83,84
Numerous studies on subjects with COPD have demonstrated that pulmonary rehabilitation improves dyspnea and health status, and, to a lesser extent, physical activities, self-reported activities of daily living, self-efficacy, and medical consumption.84 Exercise training is generally considered to be the cornerstone of a pulmonary rehabilitation program for patients with COPD and is the best way to improve their muscle function. Improvements in skeletal muscle function after exercise training lead to improvements in exercise capacity and symptoms85 and reductions in hospital admissions.86 The effects of pulmonary rehabilitation on daytime physical activity appear to be small.87
In 2000, one of the first studies investigating the value of nocturnal NIV in addition to rehabilitation was published.88 Forty-five subjects with severe stable COPD (mean FEV1 1.0 L) were randomized either to a combination of domiciliary NIV and exercise training (n = 23) or to exercise training alone (n = 22). After an 8-week training program, a mean significant improvement of 72 m in the shuttle walk test was found in the NIV-and-exercise-training group compared with the exercise-training-alone group. In addition, a significant mean improvement between both groups on the Chronic Respiratory Disease Questionnaire of 12.3 was found, which related to a clinically important difference. This suggested that domiciliary NIV can be used successfully to augment the effects of rehabilitation in severe COPD. Interestingly, this benefit was observed while normocapnic subjects were on NIV. In addition, the adherence to NIV was low, as the mean number of hours of overnight use was 2.1 h, and 50% of the subjects were on NIV for < 2 h. Although the minimum number of hours to define adherence is not known, this is generally considered as low.
A more recently published study investigated the benefits of nocturnal ventilatory support in addition to rehabilitation in hypercapnic subjects.89 Seventy-two subjects with COPD were randomly assigned to nocturnal NIV in addition to rehabilitation (n = 37) or to rehabilitation alone (n = 35). Outcome measurements were assessed before and after the 3-month intervention period. Although the primary outcome of the Chronic Respiratory Disease Questionnaire total score improved by 15.1 points with NIV and rehabilitation, the score improved by 8.7 points with rehabilitation alone, although the difference was not statistically significant between the groups (P = .08). However, it is unclear whether the Chronic Respiratory Disease Questionnaire is the best questionnaire to use for subjects with respiratory failure. In contrast, the Maugeri Respiratory Failure Questionnaire identified significant improvements in total score and the cognition domain with the addition of NIV. Furthermore, the addition of NIV improved daytime PaCO2 (P < .01) and daily step count (mean difference of 1,269 steps/d, P < .01). This was accompanied by increased daytime minute ventilation (V̇E; mean difference of 1.4 L, P < .001). In 2011, the long-term results of this study were published and showed that the benefits increased even further over time.90
A prospective observational nonrandomized study was conducted on subjects with COPD Global Initiative for Chronic Obstructive Lung Disease stage 4.91 Forty subjects received nocturnal NIV with rehabilitation for a mean of 29 d, and their results were compared with 40 matched control subjects who underwent the same rehabilitation program. Subjects in the NIV group received ∼8 h of ventilation, with a mean IPAP of 17.5 cm H2O and a mean expiratory positive airway pressure (EPAP) of 4.5 cm H2O. Significant between-group differences were found for the 6-min walk test, FEV1, and lung hyperinflation, whereas significant within-group differences were found for blood gases and QOL in the NIV group. These positive effects were also found for normocapnic subjects, suggesting that NIV should be started early in the course of COPD.
In summary, nocturnal NIV might be an effective additional tool to augment the benefits of rehabilitation. Although positive effects have been found in several clinically relevant outcomes, several issues are still open for discussion, for example, when to start NIV, optimum pressure settings, and defining the primary goals of NIV. As long as many of these issues remain unsolved, in combination with the lack of adequately powered studies, nocturnal NIV cannot be advocated as part of routine management of patients with COPD who start a rehabilitation program.
Ventilatory Support During Exercise Training
It is generally accepted that training intensity is crucial to achieve a true physiological benefit in patients: minimum increases in maximum exercise capacity are observed following low-intensity exercise training, whereas high-intensity training improves maximum and submaximum exercise and induces cardiorespiratory and peripheral muscle adaptations. Extreme breathlessness and muscle fatigue may limit the ability of patients with severe COPD to train at the recommended high levels of exercise. This may prevent the intended physiological improvement for these patients. Additional interventions in standard multidisciplinary pulmonary rehabilitation have been proposed to maximize the effect of the intervention in patients with severe COPD. The most frequently used adjuncts are optimization of bronchodilator therapy, inspiratory muscle training, supplementation with anabolic steroids and growth hormone, administration of oxygen and helium-hyperoxic gas mixtures, breathing strategies, neuromuscular electrical stimulation, walking aids, and NIV via mask or mouthpiece.83,84,92
Laboratory evidence shows that CPAP, pressure support ventilation, and proportional assist ventilation improve breathlessness and exercise tolerance in subjects with COPD by unloading the respiratory muscles while increasing ventilation.93,94 A subsequent systematic review concluded that NIV application during exercise in subjects with COPD resulted in immediate improvements in exertional dyspnea and exercise endurance.95 This effect was obtained mainly with pressure support ventilation, with smaller effects with CPAP and proportional assist ventilation.
These immediate effects of NIV on exercise-related dyspnea and exercise endurance have prompted several research groups to investigate the effects of NIV during training sessions on long-term outcomes of pulmonary rehabilitation programs in subjects with COPD.96–103 This topic has been further addressed in 3 systematic reviews.104–106 In all studies, the included study population was small (generally 7–10 per group and study). Moreover, a considerable heterogeneity between studies makes it difficult to draw firm conclusions from the published data. Subjects also differed in their pathophysiological characteristics (mean FEV1 varied between 26 and 48% of predicted) and in the cause of exercise limitation. Different ventilator settings and modes were used, and training varied from 12 to 24 sessions. Considerable differences existed between studies in training schedules, which consisted either of treadmill or cycle training, occasionally completed with either upper- or lower-limb training. During the rehabilitation sessions, training intensity was higher in the NIV group,98,102,103 whereas ventilatory requirements decreased.96,98,101 Long-term physiological outcomes, such as maximum work load assessed at the end of the program,99,102,103,106 improved significantly in favor of the NIV group, with a difference of 17% compared with the control group. Conversely, differences in endurance exercise capacity, assessed with a constant work rate exercise ergometer test, failed to reach statistical significance between the intervention and control groups.106 Likewise, training with NIV did not affect the 6-min walk distance,103 exercise dyspnea,97,102,103 or QOL.98,103 Dropouts were similar between ventilated and nonventilated subjects. Physical activity was not assessed as an outcome in any of the published studies.
In a systematic review, Ricci et al105 concluded that although NIV beneficially affected heart rate and oxygen consumption, these effects were not statistically significant. Similarly, Corner and Garrod104 suggested that NIV may allow increased exercise intensity and duration during pulmonary rehabilitation in subjects with moderate-to-very-severe COPD, making it reasonable to propose this treatment for patients with severe COPD. Finally, the authors of a Cochrane Review concluded that NIV during lower-limb exercise training may allow subjects with COPD to exercise at a higher intensity than subjects without NIV, and some evidence suggests that NIV during exercise training improves the percentage change in peak and endurance exercise capacity.106 However, the authors remarked that these findings are not consistent across other measures of exercise capacity, questioning whether these relative benefits of NIV during exercise training are clinically worthwhile and cost-effective.
Monitoring of NIV
NIV is predominantly administered during sleep. Sleep greatly influences ventilatory behavior by inducing modifications of ventilatory control, upper-airway patency, and respiratory muscle recruitment, in particular in patients with respiratory insufficiency. Therefore, NIV settings chosen empirically during the daytime may not predict optimum nocturnal ventilatory support. Consequently, NIV effectiveness might be more correctly assessed during sleep than during the daytime.107
Patients on NIV could be considered as adequately ventilated when the ventilator provides a proportional assistance to their needs without limiting the expression of respiratory activity. Additional criteria include signs of improvement or correction of alveolar hypoventilation, along with an improvement or at least a preservation of sleep quality (Table 1).108 However, until now, neither a unified definition of effective ventilation nor a strategy to evaluate its effectiveness was established. The optimum monitoring of patients on long-term NIV is still a matter of debate. Physicians caring for these patients may vary greatly in their methods of monitoring NIV, from a single blood gas measurement to full PSG.
Therapeutic Goals of NIV
Oximetry and Capnography
As already mentioned, ventilator settings established when the patient is awake may not be sufficient to improve nocturnal alveolar hypoventilation. Therefore, it has been suggested that NIV evaluation may be performed by monitoring nocturnal SpO2.109 There is an agreement on SpO2 recording as a minimum prerequisite, but some studies have shown that overnight monitoring of transcutaneously measured partial pressure of carbon dioxide (PtcCO2) is also indicated, as subjects may remain hypercapnic despite normal SpO2.110,111 PaCO2 sampling is performed mostly after an arousal or awakening and thereby followed by a period of appropriate breathing, not reflecting the abnormal breathing during sleep. Nocturnal PtcCO2 monitoring should therefore be considered as a reliable alternative, as it shows good accordance with PaCO2 measurement.112 A lag time (± 2 min) was observed in PtcCO2 measurement, not always indicating rapid changes in CO2 measurement due to respiratory events or leaks at the correct time.113 In addition, simplified built-in monitoring systems coupled with some ventilators may allow interesting additional data to be collected.114–116 A plugged interface permits SpO2 and PtcCO2 data to be obtained during the same recording. Furthermore, obtaining derivations from pulse plethysmographic parameters can provide useful autonomic markers of sympathetic tone and information on sleep fragmentation.117–121
PSG
These data (SpO2 and PtcCO2) lack some critical signals (eg, thoracoabdominal movements), which is why some authors suggest that this strategy may not be sufficient.107,108,122 Combined with flow and pressure recordings, thoracoabdominal signals are crucial to understand patient-ventilator synchrony. Recognizing thoracoabdominal movements without synchronous pressurization is a good marker of unrewarded inspiratory efforts.108 Additionally, a qualitative estimation of the effectiveness of ventilation can be obtained from these signals. Thus, when these signals are recorded together, an accurate picture of the synchronization between patient and ventilator and of the efficacy of NIV can be obtained. Using full PSG gives additional information on sleep efficiency and sleep architecture during NIV treatment and could provide more information on the occurrence of specific respiratory events during different sleep stages.
These data, together with a patient's clinical status, allow the quality of NIV to be determined. Frequently used therapeutic goals include clinical improvement plus reduction of daytime PaCO2, mean nocturnal SpO2 of > 90%, > 90% of the recording time without residual oscillations, and use of nocturnal NIV for > 4 h without discomfort (fragmented use or multiple short periods of ventilator use).123
Built-In Ventilator Software
Home ventilators have evolved rapidly since the first cohort studies, with increased use of pressure-preset ventilators over the past 20 years.124 The latest generation of home ventilators has built-in software that provides the clinician with potentially valuable information, such as adherence, estimation of leaks, tidal volume (VT), V̇E, breathing frequency, percentage of breaths triggered by the patient, percentage of pressurizations interrupted by the patient (cycling), and apnea and/or apnea-hypopnea index. Rabec et al116 were the first to evaluate the validity (reliability) of data collected by built-in software in a home ventilator and found that machine-derived leak and V̇E data highly correlated with objective laboratory-based measures of these parameters.
Estimation of VT.
To date, 2 publications have evaluated VT monitored by built-in software.114,125 These studies showed an underestimation of VT for the 11 ventilators tested. The ability of a home ventilator to maintain a stable VT is related to the pressurization capacities of the device,126 as well as to its assessment of VT, especially in the presence of leaks.127 One study showed that 4 of 6 devices designed to maintain a preset VT tended to underestimate VT.127 Also, all but one of these devices failed to maintain a preset VT in the presence of unintentional leaks. Furthermore, underestimation of VT increased significantly at higher pressure support levels.114 Overall, the bias in VT ranged from 66 to 236 mL, thus introducing a considerable possibility of error in adjusting ventilator settings. As data provided by software tend to underestimate VT, this can lead clinicians to increase pressure support, which can in turn aggravate leaks.
Estimation of Leaks.
Different devices do not estimate leaks in the same way. One study showed an important variability in the estimation of leaks.114 Simulating a continuous leak over a long period in standard home ventilators generally revealed an underestimation of leaks. In this case, precision of leak estimation varied significantly between devices. In some ventilators, bias for estimation of leaks clearly increased with significant unintentional leaks. In a recent publication, Sogo et al128 generated a leak during the inspiratory phase, which more closely mimics a clinical situation. In this study, the 4 commercial tools overestimated unintentional leaks.
In conclusion, data provided by ventilator software can be a useful adjunct and an important contribution to monitoring long-term domiciliary NIV.123 However, the clinician must be aware of the lack of standardization in the reporting of collected data and the variable reliability of results provided according to the device used. Items that have not yet been independently assessed, such as apnea and apnea-hypopnea indexes, need to be further evaluated both in bench studies and clinically by comparison with PSG. Finally, a consensus between manufacturers on measuring and reporting data would be helpful. Alternatively, as discussed by Luján and Pomares,129 the development of independent monitoring tools would better assist clinicians.
Leaks and Patient-Ventilator Asynchrony
Leaks are inherent to NIV. They are divided into intentional leaks (ie, those associated with the exhalation valve either placed in the tubing or built into the interface) and unintentional leaks (ie, leaks occurring anywhere between the ventilator and the patient's airways, but not through the exhalation valve). Intentional leaks are mandatory for elimination of CO2 from the ventilation circuit and avoiding rebreathing. They can vary considerably from one interface to another, and choice of interface may affect the capacity to achieve preset pressure support.130 Unintentional leaks always occur to some extent during NIV. Bi-level pressure support ventilators or ICU ventilators with an NIV mode are designed to detect and compensate for these leaks.
Patient-ventilator asynchrony refers to the presence of an asynchrony between the patient's neural respiratory drive and effective ventilation or pressurization.108 It encompasses respiratory events such as ineffective or unrewarded inspiratory efforts, autotriggering, double or multiple triggering, and prolonged dissociation between pressurizations and inspiratory efforts. Intracycle patient-ventilator asynchrony (ineffective or delayed triggering, premature or late cycling) may also result from unintentional leaks.131 These events have been shown to affect sleep structure and may affect work of breathing.132,133
Patient-ventilator asynchrony has been observed to increase the microarousal index and stage 1 and 2 sleep and to decrease slow-wave and rapid-eye-movement sleep in subjects with stable obesity-hypoventilation syndrome on long-term NIV.133 Ineffective efforts may also affect efficacy of NIV and lead to deterioration in nocturnal gas exchange and arterial blood gases.132
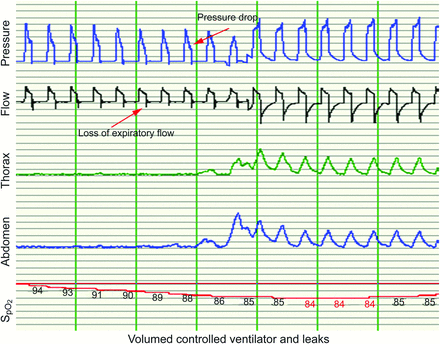
Leaks have a clinically relevant deleterious impact in NIV.134–136 In volume-cycled NIV, they lead to insufficient compensation of hypoventilation, lower VT, and lower PEEP and affect the ability of the patient to trigger the ventilator (Fig. 1). A study on pressure-preset NIV showed that unintentional leaks disrupted sleep architecture; increased the arousal index and PtcCO2; and decreased total sleep time and slow-wave and rapid-eye-movement sleep.135 Leaks may decrease the FIO2 when supplemental oxygen is administered during NIV and may lead to significant pressure drops, poor inspiratory triggering, increases in duration of inspiratory pressurization, and even inversion of the inspiratory-expiratory ratio.135–137 Pressure drops induced by unintentional leaks depend on the ventilators tested because leak compensation varies markedly from one ventilator to another (Fig. 2).138 In fact, leaks may suffice to explain residual nocturnal hypoventilation as has been documented in subjects on NIV for respiratory failure resulting from neuromuscular disorders.139
Leaks lead to disappearance of thoracic and abdominal movements, a major decrease in tidal volume, and desaturation.
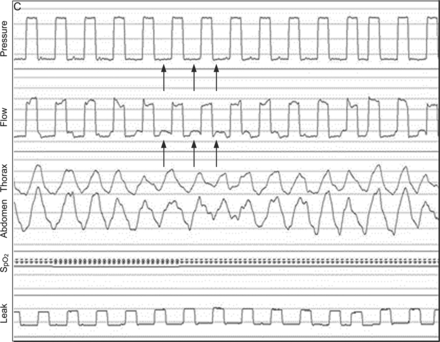
Traces show a significant increase in ventilator flow to compensate for leaks, inversion of inspiratory flow tracing, and decreases in inspiratory pressure and thoracic and abdominal movements.
Leaks and patient-ventilator asynchrony are related: in ICU subjects, leaks have been shown to be significantly correlated with ineffective breath efforts, delayed cycling, and presence of an asynchrony index above 10% of the total recording time.131 Conversely, using an NIV algorithm in ICU ventilators significantly decreases the impact of leaks on patient-ventilator asynchrony.140 In subjects on long-term NIV for neuromuscular disorders, patient-ventilator asynchrony was shown to occur in relation to leaks.141 In this setting, patient-ventilator asynchrony events were mainly ineffective inspiratory efforts, autotriggering, and prolonged insufflations. Patient-ventilator asynchrony rates were correlated with arousals and awakenings.
Bench tests have demonstrated a clear relationship between induced unintentional leaks and delayed cycling (leading to prolonged insufflations and even inverted inspiratory-expiratory ratios).138 More recently, 9 ICU ventilators were tested with increasing levels of leaks: all were able to maintain their VT, but several required adjustment of triggering or cycling criteria, and some switched to backup breathing frequency.142
In summary, unintentional leaks are frequent during NIV. They have a major impact on ventilation efficacy in volume-cycled devices that do not compensate for these leaks. In bi-level positive airway pressure ventilation (or NIV mode for ICU ventilators), these leaks are partially or totally compensated according to the devices used. However, unintentional leaks are associated with patient-ventilator asynchrony. Patient-ventilator asynchrony may in turn affect NIV efficacy and sleep structure, although considerable patient-ventilator asynchrony may occur without any adverse effect on arterial blood gases and correction of nocturnal hypoventilation.133 The relationship between sleep disruption resulting from patient-ventilator asynchrony, leaks, and clinical outcomes such as adherence, HRQOL, or even survival requires further evaluation.
Identifying Respiratory Events During NIV
Previous observations have shown that standard definitions for nocturnal respiratory events in spontaneous breathing do not lend themselves well to the description of respiratory events occurring during positive-pressure ventilation. Indeed, one major difference during NIV is the continuous interaction between the ventilator, generating an intermittent positive pressure, and the patient's neural respiratory drive.107 During NIV, the patient is assisted by a ventilator, and events can result from the patient, the ventilator, or poor patient-ventilator synchrony. These different events have been described by Gonzalez-Bermejo et al108 (Table 2).
Respiratory Events During NIV
A respiratory event is defined as the occurrence of a modification, discontinuity, or instability of ventilation that has deleterious consequences on SpO2, PtcCO2, and/or sleep. The SomnoNIV Group considered the presence of signals for pressure, flow, abdominal and thoracic movements, and SpO2 as a minimal prerequisite for analysis of these events.108
Few studies have tried to relate these respiratory events to alterations in sleep structure and/or reductions in SpO2. Studying a pediatric population on long-term NIV, Caldarelli et al143 demonstrated that respiratory events were common even in compliant children who did not complain of any symptoms. A recent study analyzing 125 polygraphies during NIV in adults demonstrated that the mean time spent overnight with respiratory events was > 20%.144 Time spent with respiratory events significantly correlated with lower nocturnal SpO2 and higher diurnal PaCO2. In both studies, pathophysiology-based adjustment of ventilator settings led to a significant improvement in the quality of ventilation.143,144 This emphasizes the benefit of performing PSG in ventilated patients to optimize NIV efficacy.
Unintentional Leaks.
As discussed above, the impact of leaks on ventilatory efficacy depends not only on the absolute amount of leaks, but also on the capability of the device to compensate for them.114,125 Furthermore, the influence of leaks could vary with the underlying pathology (ie, the difference in respiratory mechanics).144 Therefore, detecting unintentional leaks and particularly their impact on quality of ventilation is of major importance when monitoring NIV.
The importance of leaks and the ability of the ventilator to compensate for them determine whether the pressure signal amplitude remains stable or decreases.108 A fall in positive pressure (inspiratory and expiratory) indicates major unintentional leaks. With pressure control ventilators, an increase in ventilator flow signal during insufflation with a simultaneous decrease in thoracic and abdominal belt signal amplitude is suggestive of unintentional leaks (Fig. 3). Ventilator flow increases to compensate for the drop in pressure, but leaks result in an effective reduction in VT delivered to the patient. Because of the inability of volume control ventilators to compensate for leaks, a decrease in thoracic and abdominal belt signal amplitude can occur even in the presence of small leaks without any increase in flow signal. However, a decrease in the pressure signal is usually observed. Furthermore, as target-volume ventilation is usually provided by a circuit in which inspiration and expiration are separated by a true expiratory valve, a sharp reduction in the expiratory part of the flow curve indicates the loss of expiratory flow in the circuit and thus suggests the presence of leaks (Fig. 4).
Typical patterns: increase in machine flow amplitude, reduction in thoracic and abdominal belt signals, and mild reduction in inspiratory pressure. Unintentional leaks as estimated by the ventilator software coupled to the polygraph are represented at the bottom of the graph.
Inspiratory pressure is not maintained (arrows). Inspiratory flow amplitude is maintained with reduction in thoracic and abdominal belt signals. Note the amputation of the expiratory part of the flow curve (arrows).
Decrease in Ventilatory Drive.
During sleep, the voluntary controller is abolished, and ventilation becomes exclusively dependent on automatic control. In addition, ventilatory control is physiologically altered during sleep, with a decreased responsiveness to chemical, mechanical, and cortical inputs. If NIV settings lead to hyperventilation, bursts of central apnea or hypopnea can occur, particularly during transitions between sleep onset and wakefulness.145 Adduction of the vocal cords resulting in progressive closure of the glottis has also been described in response to ventilator-induced hyperventilation.147
The essential feature of a decrease in ventilatory command is a proportional and simultaneous reduction in flow and thoracic and abdominal belt signal amplitudes without phase opposition.108 In the absence of a backup breathing frequency (ventilator in spontaneous mode), thoracic and abdominal belt signals may disappear completely, generating a pattern of recurrent central apneas (Fig. 5A). With a backup breathing frequency, the length of events is limited by the preset maximum interval between breaths (Fig. 5B).
A: In the absence of backup ventilation: loss of pressure signal, flow signal, and thoracic and abdominal belt signals. Shaded areas denote desaturations. B: With backup breathing frequency: reduction in abdominal and thoracic belt signal amplitudes without phase opposition. Note the switch to backup ventilation.
Partial or Total Upper-Airway Obstruction With or Without Reduction in Ventilatory Drive.
Intermittent obstruction of the upper airway is common during NIV and may be related to 2 mechanisms. The first corresponds to obstructive events at the oropharyngeal level because of upper-airway collapse as a result of insufficient EPAP. This mechanism may be present in patients with an unstable upper airway, such as patients with obstructive sleep apnea or obesity-hypoventilation syndrome. The second mechanism corresponds to episodes of intermittent obstruction at the glottic level, reflecting cyclic glottic closure induced by hyperventilation. Positive-pressure ventilation-induced hyperventilation has been shown to promote active glottic closures in normal subjects when awake or asleep.147 Glottic closure was shown to be proportional to total ventilation and inversely proportional to end-tidal CO2. By using simple tools such as nocturnal SpO2, these mechanisms are indistinctly expressed as desaturation dips during NIV because SpO2 cannot distinguish between apneas at oropharyngeal and glottic levels.116
Moreover, both mechanisms are located at different levels in the airway and represent different pathophysiological mechanisms. They also have a distinct semiology in the curves, and their therapeutic approach is different. Although both cases are characterized by a sudden reduction in flow amplitude during insufflation, obstruction at the oropharyngeal level will be accompanied by progressively increased inspiratory activity, indicating a struggle against upper-airway collapse. This will be expressed as a progressive increase in abdominal and thoracic belt signals, with or without a phase opposition or a phase angle between thoracic and abdominal belt signals, suggesting partial or total closure of the upper airways (Fig. 6A).108 In this case, the strategy is to increase the level of expiratory pressure to stabilize the upper airway.
A: Without reduction of ventilatory drive: sudden reduction in flow amplitude, phase opposition or phase angle in thoracic and abdominal belts, increase in breathing frequency at the end of the event due to increase in patient efforts to open the airways, and patient-ventilator asynchrony. Shaded areas denote desaturations. B: With decrease in ventilatory command: progressive decrease in flow, pressure signal unchanged, disappearance of thoracoabdominal movements and switch to backup breathing frequency without thoracoabdominal movements (indicating recurrent airway closure with reduced ventilatory command), and entirely synchronous resumption of flow and thoracoabdominal movements without fighting movements.
Conversely, if the underlying mechanism is glottic closure, the essential feature of a decrease in ventilatory command is a decrease in flow with a simultaneous reduction or disappearance of thoracic and abdominal belt signals, which occurs without phase opposition as a result of an excessive level of ventilation promoting respiratory pauses (Fig. 6B).108 In this case, the suggested approach is to reduce V̇E.147,148 Once again, this emphasizes analysis of thoracoabdominal belts to assess NIV quality and guide therapy.
Asynchrony.
Synchrony between a patient's spontaneous breathing activity and the ventilator's parameters is one of the key factors determining efficacy and tolerance to NIV. Asynchrony during triggering and cycling is quite common during sleeping patients on long-term NIV. According to different published series, the estimated prevalence of significant asynchronies varies from 17 to 55%.132,133,143 Periods of desynchronization are frequently associated with arousals.133 Although no increase in PtcCO2 is observed during asynchronies,133 data on nocturnal SpO2 measurement are divergent.132,133 During NIV, leaks may greatly affect patient-ventilator synchrony, and most asynchronies in subjects on NIV are related to unintentional leaks.140 Different types of leak-induced asynchronies are shown Figure 7. It has been suggested that patient-ventilator synchrony should be monitored nocturnally in all patients starting long-term NIV to identify those for whom a change in ventilator settings would be beneficial.149,150 Future studies should focus on whether the presence of these asynchronies and minimization of these events have an effect on the efficacy of ventilation, adherence, QOL, and survival.
Example of leak-induced asynchrony during pressure control ventilation. A: Autotriggering is defined as the occurrence of at least 3 consecutive pressurizations at a ventilator frequency of > 40/min not synchronized with patient inspiratory effort as defined by Guo et al.132 B: Double triggering is defined as 2 cycles separated by a very short expiratory time, defined as less than half of the mean inspiratory time and concomitant inspiratory activity at the thoracoabdominal bands. C: Ineffective inspiratory efforts are defined by the presence of a respiratory movement on thoracoabdominal bands and/or a positive deflection in expiratory flow (arrow), without a concomitant breath delivered by the ventilator (arrows). In this example, as leaks impede the detection of patient inspiratory effort, the ventilator switches to backup breathing frequency. Unintentional leaks as estimated by the ventilator software coupled to a polygraph are represented at the bottom of each graph.
NIV Modes: Recent and Future Developments
Target-Volume NIV
Generally, there are 2 modes of NIV that can be applied: pressure- and volume-preset NIV. During pressure-preset NIV, a fixed IPAP is provided by the ventilator, and the VT can vary depending on chest-wall and lung compliance. In contrast, during volume-preset NIV, a fixed inspiratory volume is set using a variation of IPAP.151 In the past, both modes of NIV have been successfully used in different pathologies, with comparable improvements in gas exchange, lung function, sleep quality, and HRQOL.152,153
To combine the advantages of pressure- and volume-preset NIV, a hybrid mode called target-volume NIV was introduced over 10 years ago.151 In this mode, a preset target volume (per breath or per min) and 2 levels of IPAP (minimal and maximal) are combined. Several studies have been performed with target-volume NIV in different subject cohorts (Table 3). In one study by Storre et al,154 target-volume NIV reduced nocturnal PtcCO2 more than the conventional mode of pressure-preset NIV in subjects with obesity-hypoventilation syndrome. This finding was confirmed by Janssens et al,155 but sleep quality was found to be worse with target-volume NIV, possibly due to IPAP variations during sleep. Interestingly, the opposite results were detected by Crisafulli et al156 in subjects with COPD, in whom a better subjective sleep efficiency was reported with target-volume NIV compared with conventional NIV. Thus, the effect of target-volume NIV on sleep quality currently remains unclear.
Clinical Studies on Target-Volume NIV
The first trials reported that target-volume NIV is superior to conventional NIV in reducing nocturnal PCO2.154,155,157 However, 3 more recent trials could not confirm this advantage (Table 3).158–160 Murphy et al159 reported comparable results regarding daytime PCO2, QOL, weight loss, and improvements in Epworth Sleepiness Scale scores after a 3-month RCT in subjects with obesity-hypoventilation syndrome. Importantly, in this trial, an individual adjustment of ventilator settings according to a predefined protocol with inclusion of a nocturnal assessment period and aiming to achieve optimum nocturnal respiratory control was applied. Two other studies investigated target-volume versus pressure-preset NIV in subjects with chronic hypercapnic COPD.158,160 In line with the results of the previous study,159 control of daytime and nocturnal hypoventilation, HRQOL, lung function, and exercise capacity were similar between these 2 modes.158,160 A possible explanation for the disparity between the earlier studies and the more recent ones may be that protocolized NIV titration was performed in one study,158 and higher inspiratory pressures were applied in the pressure-preset groups.158–160
In 2 recently published bench studies, the effect of unintentional leaks on target-volume ventilation was investigated under different conditions. In a study by Khirani et al,161 single-limb circuits with intentional leaks estimated the expiratory target volume more accurately compared with single circuits with an expiratory valve or ventilators using a double circuit. Additionally, Carlucci et al162 reported that vented circuits are able to better compensate for leaks compared with nonvented circuits with an active valve.
In conclusion, target-volume NIV showed similar clinical benefits compared with the conventional treatment of pressure-preset NIV, but general recommendations for its application cannot be given. If target-volume NIV is used, a single-limb circuit with intentional leaks is recommended.
Electromyogram-Triggered NIV
Although the neural output of the brainstem cannot be measured directly in humans, neural respiratory drive can be assessed indirectly by an electromyogram (EMG) of the respiratory muscles. Neural respiratory drive provides a breath-by-breath measurement of the balance between the respiratory muscle load and capacity. Respiratory muscle EMG measurements have demonstrated reproducibility in healthy subjects and subjects with COPD, cystic fibrosis, asthma, obesity, and motor neuron disease and subjects requiring mechanical ventilation.163–169
The accepted standard method for quantifying neural respiratory drive is the diaphragm EMG using multipair esophageal electrodes attached to a nasogastric catheter positioned across the diaphragm crus.170–172 Diaphragm EMG has been used to quantify the respiratory muscle load-capacity balance and to identify the timing of inspiratory effort. The diaphragm EMG signal has also been incorporated into mechanical ventilation to improve patient-ventilator asynchrony and to optimize the delivery of pressure support. This mode of mechanical ventilation is termed neurally adjusted ventilator assist.173,174
Conceptually, using neural respiratory drive to trigger NIV has advantages. The diaphragm EMG signal is independent of interface leaks and intrinsic PEEP (observed in subjects with obstructive lung disease), both of which impact trigger performance.168,174–176 Furthermore, using neural respiratory drive to detect patient-ventilator asynchrony may lead to improvements in outcomes, including respiratory muscle unloading, gas exchange, comfort, and sleep quality.131,132,149,177 Quantifying neural respiratory drive using diaphragm EMG is challenging, however, in awake and nonsedated subjects due to the invasive nature of catheter insertion. The operator must be experienced in placing the catheter in a conscious subject to acquire a diaphragm EMG signal, and there are also concerns that the signal may be affected by a cardiac artifact, changes in body position, and lung volume.178,179
The second intercostal space parasternal intercostal muscles are obligate inspiratory muscles that contract in concert with diaphragm muscles during inspiration to stabilize the upper chest wall.180–182 Adjacent to the sternum, second intercostal space parasternal intercostal muscles have inspiratory mechanical advantage with little postural artifact, and mapping the neural respiratory drive of the chest-wall muscles has confirmed this to be the point of maximum inspiratory neural respiratory drive.181,182 The timing of the peak parasternal intercostal muscle EMG is similar to diaphragm EMG, and it thus can be considered as a noninvasive surrogate.183,184 Parasternal intercostal muscle EMG has also been correlated with breathlessness, with changes in diaphragm EMG activity with threshold and hypercapnic loading.167,185 Parasternal intercostal muscle EMG is suitable for overnight monitoring of respiratory muscle activity in subjects with asthma and subjects on NIV.165,186
Previous work used surface diaphragm EMG measurements to identify patient-ventilator asynchrony during NIV. However, this is prone to interference with crosstalk from abdominal muscle groups.130,178,182 Alternative markers of inspiratory effort include respiratory inductance plethysmography. However, respiratory inductance plethysmography may underestimate the prevalence of patient-ventilator asynchrony if neural respiratory drive is insufficient to result in chest-wall expansion or if there is a delay between onset of neural respiratory drive and chest-wall excursion. A combination of parasternal intercostal muscle EMG and respiratory inductance plethysmography has been proposed to identify the type and frequency of patient-ventilator asynchrony, with adequate interobserver reliability demonstrated.187
Parasternal intercostal muscle EMG allows quantification of respiratory muscle loading and unloading, which may enhance the understanding of ventilator modes and settings under different pathophysiological conditions. In addition, parasternal intercostal muscle EMG may be used as direct feedback to optimize ventilator settings, enhance triggering, and provide a patient-centered approach to set up and deliver NIV. If parasternal intercostal muscle EMG is to drive NIV, then an understanding of the attenuation in peak parasternal intercostal muscle EMG activity during different sleep stages must be obtained to ensure adequate overnight pressure support without the risk of hypoventilation. Conditions in which neural respiratory drive would not be useful to drive NIV require clarification, such as metabolic acidosis, when elevated levels of neural respiratory drive could induce hyperventilation and indeed risk lung injury.188 Finally, for parasternal intercostal muscle EMG to be used routinely, acquisition, processing, and analysis of the signal must be automated and inform the clinician of the neural respiratory drive level and type and proportion of patient-ventilator asynchrony and differentiate between EMG-triggered and ventilator-delivered breaths.
Autoregulating Algorithms
Central breathing disturbances may appear due to 2 different pathophysiological situations. Central sleep apnea may be associated with hypercapnia in patients with a reduction in respiratory drive (ie, hypoventilation); however, central sleep apnea may emerge due to hyperventilation (non-hypercapnic central sleep apnea). These breathing disturbances appear at high altitude in subjects with heart or renal failure or acromegaly or without known predisposition or risk factors (idiopathic central sleep apnea).
An increasing number of patients who suffer from both sleep-related breathing disorders and cardiovascular diseases have been identified. The Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial demonstrated that treatment with CPAP failed to sufficiently treat half of the subjects with central sleep apnea and Cheyne-Stokes respiration due to cardiovascular diseases.189 However, a post hoc analysis suggested that optimum suppression of central sleep apnea in cardiac subjects might improve survival.190
ASV represents an algorithm that automatically varies the pressure support on a breath-by-breath analysis to decrease the ventilatory over- and undershoot of the respiratory system in periodic breathing. Several studies showed that ASV is superior to CPAP in terms of suppressing respiratory disturbances and improving left-ventricular function in subjects with heart failure.191,192 A multinational multi-center randomized parallel trial (SERVE-HF) is ongoing and will provide important data on the effect of ASV treatment on morbidity and mortality in subjects with chronic heart failure and central sleep apnea/Cheyne-Stokes respiration.193
However, the question remains as to whether ASV should be used in only patients with Cheyne-Stokes respiration. Allam et al194 retrospectively analyzed 100 subjects with different phenotypes of central disturbances, including central sleep apnea, Cheyne-Stokes respiration, and CPAP-emergent complex sleep apnea. They found that ASV was superior to bi-level positive airway pressure in spontaneous and spontaneous-timed mode, CPAP, and CPAP plus oxygen. In addition, Morgenthaler et al195 compared the efficacy of NIV and ASV in 21 subjects with central sleep apnea/Cheyne-Stokes respiration, mixed apneas, and complex sleep apnea. Although both treatment options improved respiratory disturbances, arousals, and sleep profiles, ASV was significantly superior to NIV.
Most recent algorithms combine auto-adjusting EPAP, expiratory pressure relief, and ASV. They automatically vary the expiratory pressure to adapt to different levels of upper-airway obstruction, reduce pressure during early expiration to facilitate patient respiration, and variably adapt pressure support to overcome periods of central sleep apnea/Cheyne-Stokes respiration. There is a lack of high-level evidence confirming the superiority of these enhanced algorithms compared with conventional ASV with fixed, manually titrated EPAP, although results from pilot studies show a possible benefit on the control of respiratory events in subjects with central sleep apnea.196,197
However, a clear differentiation of hypoventilation and hyperventilation disorders is crucial for optimum treatment. A general principle of the adaptive algorithm is the continuous comparison of the V̇E or VT with target parameters in a moving window gliding throughout the night. The reaction of the ASV algorithms might be diminished during long-term periods of hypoventilation, which may lead to misdiagnosis due to the moving window. Hence, it is not appropriate to use ASV in hypoventilating patients (ie, patients with neuromuscular or lung disease and daytime hypercapnia). As discussed above, the use of target-volume ventilation could be considered for these patients if there is a variable need for pressure support.
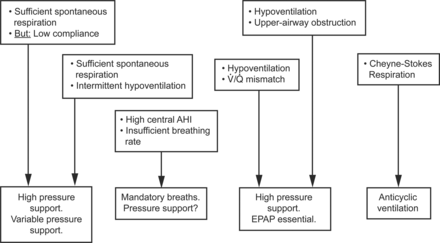
Thus, requirements of autoregulating algorithms depend on different comorbidities. (1) Coexisting obstructive sleep apnea and central sleep apnea are characterized by variable levels of obstruction, with a risk of emerging complex sleep apnea upon positive airway pressure application. (2) Subjects with extreme obesity require high treatment pressures to overcome upper-airway obstruction. However, ventilation varies according to body position and central respiratory drive. (3) Neuromuscular diseases are associated with continuous hypoventilation due to low VT and increased upper-airway collapsibility. (4) Obstructive lung disease is characterized by dynamic hyperinflation and increased lung compliance. These different situations require different therapeutic responses (Fig. 8).
Therapeutic algorithm. AHI = apnea-hypopnea index; V̇/Q̇ = ventilation/perfusion; EPAP = expiratory positive airway pressure.
In conclusion, hyperventilation disorders may be associated with cardiac diseases, chronic hypocapnia, reduced CO2 reserve, increased central and peripheral chemosensitivity, and changes in acid/base balance. CPAP is generally recommended as the first therapeutic approach, but it fails in ∼50% of the patients. ASV has proven to be superior to CPAP in terms of respiratory disturbances, sleep, and cardiac parameters. Coexisting obstructive sleep apnea and hypoventilation syndromes are associated with reduced respiratory drive, upper-airway obstruction, and reduced thoracic compliance. If CPAP fails, NIV can be applied. However, algorithms that combine automatic CPAP, respiratory pressure relief, and pressure support should be studied systematically in these complicated breathing patterns.
Conclusions
NIV is a well-established therapeutic option for chronic respiratory failure due to a variety of underlying diseases. The report of this 2-day working group discusses several questions regarding the management of patients with NIV, and the following conclusions can be made. (1) Early initiation of NIV in ALS seems to provide beneficial effects, but its use in combination with secretion management is of major importance. The effect of NIV on sleep in patients with ALS needs further research. (2) Long-term invasive ventilation could improve survival, but as it is a very expensive treatment with high burden of care, early discussion about this decision is recommended. (3) In patients with COPD, high-intensity NIV seems to be associated with better outcomes compared with low-intensity NIV. (4) NIV in combination with or during exercise training has beneficial effects in patients with COPD, but more research on this topic is needed. (5) Monitoring of NIV has evolved during the last decades. Currently, polygraphy seems to be the minimum tool required to provide detailed information about the patient, the ventilator, and their interaction. The new-generation ventilators with built-in software can provide additional information on NIV use in the home setting, although data quality can differ between different manufacturers. (6) More studies are needed on the advantages of target-volume NIV in different subject populations. (7) EMG-triggered NIV could be a new feature to optimize ventilation, but its incorporation in daily use and its goals still need to be defined. (8) ASV seems to have found a place in the treatment of central sleep apnea/Cheyne-Stokes respiration.
Additional work is required to clarify the optimum NIV modes and settings for specific diseases and patients. In future research, we should be mindful that the ultimate goal is not only to improve survival, but also sleep quality and HRQOL.
Footnotes
- Correspondence: Dries Testelmans MD PhD, Department of Respiratory Diseases, University Hospitals Leuven, Herestraat 49, B-3000 Leuven, Belgium. E-mail: dries.testelmans{at}uzleuven.be.
Dr Chatwin has disclosed relationships with ResMed, Covidien, and B&D Electromedical. Dr Randerath has disclosed relationships with Weinmann, Philips Respironics, Heinen + Löwenstein, and Inspire. Dr Storre has disclosed relationships with Philips Respironics and Breas Medical. Dr Windisch has disclosed relationships with Maquet, Dräger, Heinen + Löwenstein, Philips Respironics, Weinmann, ResMed, Covidien, Linde, Siare, and Breas. Mr Vrijsen was supported by the Clinical Research Foundation of UZ Leuven (Leuven, Belgium). The other authors have disclosed no conflicts of interest.
- Copyright © 2015 by Daedalus Enterprises
References
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.
- 52.↵
- 53.↵
- 54.↵
- 55.
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- 64.
- 65.
- 66.↵
- 67.↵
- 68.↵
- 69.↵
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.↵
- 75.↵
- 76.
- 77.↵
- 78.↵
- 79.↵
- 80.↵
- 81.↵
- 82.↵
- 83.↵
- 84.↵
- 85.↵
- 86.↵
- 87.↵
- 88.↵
- 89.↵
- 90.↵
- 91.↵
- 92.↵
- 93.↵
- 94.↵
- 95.↵
- 96.↵
- 97.↵
- 98.↵
- 99.↵
- 100.
- 101.↵
- 102.↵
- 103.↵
- 104.↵
- 105.↵
- 106.↵
- 107.↵
- 108.↵
- 109.↵
- 110.↵
- 111.↵
- 112.↵
- 113.↵
- 114.↵
- 115.
- 116.↵
- 117.↵
- 118.
- 119.
- 120.
- 121.↵
- 122.↵
- 123.↵
- 124.↵
- 125.↵
- 126.↵
- 127.↵
- 128.↵
- 129.↵
- 130.↵
- 131.↵
- 132.↵
- 133.↵
- 134.↵
- 135.↵
- 136.↵
- 137.↵
- 138.↵
- 139.↵
- 140.↵
- 141.↵
- 142.↵
- 143.↵
- 144.↵
- 145.↵
- 146.
- 147.↵
- 148.↵
- 149.↵
- 150.↵
- 151.↵
- 152.↵
- 153.↵
- 154.↵
- 155.↵
- 156.↵
- 157.↵
- 158.↵
- 159.↵
- 160.↵
- 161.↵
- 162.↵
- 163.↵
- 164.
- 165.↵
- 166.
- 167.↵
- 168.↵
- 169.↵
- 170.↵
- 171.
- 172.↵
- 173.↵
- 174.↵
- 175.
- 176.↵
- 177.↵
- 178.↵
- 179.↵
- 180.↵
- 181.↵
- 182.↵
- 183.↵
- 184.↵
- 185.↵
- 186.↵
- 187.↵
- 188.↵
- 189.↵
- 190.↵
- 191.↵
- 192.↵
- 193.↵
- 194.↵
- 195.↵
- 196.↵
- 197.↵