Abstract
During the early phase of the COVID-19 pandemic, many respiratory therapies were classified as aerosol-generating procedures. This categorization resulted in a broad range of clinical concerns and a shortage of essential medical resources for some patients. In the past 2 years, many studies have assessed the transmission risk posed by various respiratory care procedures. These studies are discussed in this narrative review, with recommendations for mitigating transmission risk based on the current evidence.
Introduction
The SARS-CoV-2 pandemic has caused the loss of millions of human lives worldwide.1 Understanding the transmission routes and instituting appropriate measures to protect more people from acquiring the disease is crucial to contain the pandemic.2 First-line health care workers are at higher risk of infection, highlighting the importance of classifying various procedures with different levels of transmission risk.3,4 Health agencies, including the Centers for Disease Control and Prevention (CDC), have noted that specific medical procedures increase the transmission risk for respiratory pathogens because they generate aerosols.5,6 The CDC has defined aerosol-generating procedures (AGPs) as procedures that generate higher concentrations of infectious respiratory aerosols than coughing, sneezing, talking, or breathing.6 Based on this definition, the CDC classified noninvasive ventilation (NIV) as an AGP and was uncertain regarding the use of high-flow nasal cannula (HFNC) and nebulization.6 Due to the aerosol transmission concerns, clinicians tended to prefer other procedures, such as aggressive endotracheal intubation without first attempting HFNC or NIV therapy, which contributed to the critical shortage of ventilators during the early stages of the pandemic.7-9 Likewise, the restriction of nebulizer use resulted in the increased demand for metered-dose inhalers, dry powder inhalers, and soft mist inhalers, leading to a shortage of these devices in hospitals and a trend to deny therapy with medications that were not available by inhalers. This restriction of nebulizer use negatively affected the health of the patient population with chronic lung diseases, especially during exacerbations.10 Therefore, understanding the transmission risk of different respiratory treatments and appropriate methods to mitigate disease transmission by aerosols is essential.11 In the past 2 years, many studies have been published on this topic; and these findings are discussed in this narrative review, with recommendations based on the current evidence.
Aerosol-Generating Versus Aerosol-Dispersing Procedures
Distinguishing AGPs that increase the generation of bioaerosols by the patient from aerosol-dispersing procedures (ADPs) that disperse bioaerosol is essential to classify the transmission risks for different treatments, and understanding how the aerosol particles are formed during respiratory activities and their transmission routes is crucial to implementing appropriate preventive and protective measures.11-13
Patients infected with respiratory viruses produce aerosols of various sizes during coughing, sneezing, breathing, and talking.12-18 Their site of origin determines the content and size of the aerosol particles.12,18,19 Large particles (> 5 µm) are commonly produced during talking, coughing, and sneezing from the oropharynx and upper airways, whereas small particles are mostly exhaled from the bronchioles and the larynx during breathing, talking, and coughing.17 Large particles settle rapidly on surfaces and travel a short distance from the subject, whereas small particles spread further and get diluted by the surrounding air. The respiratory particles < 5 µm are referred to as droplet nuclei, and they can remain suspended in the air for prolonged periods.12,16 The viruses that are carried within these respiratory particles can transmit the infection. Thus, respiratory viruses can be transmitted through various modes, including by physical contact or as droplets and airborne particles. Droplet transmission occurs when the virus contained in the particles produced during respiratory activities comes into contact with another host’s eyes, nose, or mouth. Transmission of infection can also occur when virus-loaded droplets contaminate surfaces that then transmit to another person’s hands and then to their eyes, nose, or mouth by touching or rubbing. In contrast, airborne transmission occurs when viruses contained within droplet nuclei are inhaled by a susceptible host and then carried by the inspiratory air flow to their site of deposition within the respiratory tract.12-20
Whereas the concentration of bioaerosols in the environment is important, both distance and duration of exposure influence transmission risk.16 The distance from the source of infected aerosol generation is crucial in determining transmission risk, with the viral load and aerosol concentration increasing as the subject gets closer to the source of the emissions. Additionally, a longer duration of exposure to an infectious aerosol increases the risk of transmitting infection. Other factors that impact the spread of infection include the severity of the underlying disease (host susceptibility) and environmental factors such as ventilation and humidity in indoor spaces.12,20,21
Procedures should be classified as AGPs if they provoke cough to produce infectious bioaerosol particles that exceed levels associated with baseline activities (such as breathing or talking). Such AGPs include nasal pharyngeal suctioning, open suctioning for patients with tracheostomy, bronchoscopy examination, and intubation. In contrast, treatments are classified as aerosol-dispersing procedures if they increase the dispersion of bioaerosols exhaled by the infected person to a further distance from the patient, such as the dispersion of aerosols that occurs during the use of HFNC and NIV. These aerosol-dispersing procedures do not generate additional bioaerosol, and the consequence of dispersing the bioaerosols containing viruses depends on the viral load, aerosol particle size, and the speed and distance to which particles are transported.12,13
Methods to Evaluate Transmission Risk of Respiratory Viruses
Investigators have utilized direct and indirect methods to evaluate aerosol transmission risk.13 Direct assessment of viral load within aerosol particles requires air sampling to collect aerosol and laboratory testing by quantitative polymerase chain reaction and virus culture. However, those assessments are time consuming, expensive, and the expertise and equipment for such tests are only available in some specialized laboratories.22
Indirect technologies used to evaluate the aerosol transmission risk include aerosol particle sizers, smoke light detection, schlieren imaging, and laser light scattering.23-29 The latter 3 use imaging to visualize the trajectory of aerosol movement and do not measure aerosol particle size or concentrations.13,29 Smoke light detection uses externally introduced particles to visualize the exhaled gas from the manikin, and thus it only measures aerosol dispersion potential. In comparison, the laser light method detects any generated particles/aerosols originating from the respiratory tract and thus might measure AGP potential. The aerosol particle sizer or aerodynamic spectrometer measures particle concentrations by aerodynamic size in groups between 0.3–20 μm.13 The size and concentration measurement of aerosol particles provides valuable information, as the particle size affects the transmission routes and the concentrations should reflect the viral load.12,13 However, the particle sizer only measures the particle size and concentration at the specific distance from the source at which it is placed, and the results might be affected by other activities that impact changes in ambient gas flows, such as body movements. Moreover, an adequate interval between tests is required to clean the room air, allowing ambient particle counts to return to baseline.13,30 An important distinction between the measurements of particle concentrations and exhaled gas dispersion distance is that these measures only reflect the aerosol characteristics; but neither one directly represents the risk of infection via aerosol transmission.
Transmission Risk and Mitigation Strategies of Different Respiratory Treatments
This section will discuss the findings of studies that applied direct or indirect methods to evaluate the aerosol transmission risks of various respiratory treatments, along with appropriate mitigation strategies (Table 1).
Transmission Risk Associated With Different Respiratory Treatments and Specific Strategies to Mitigate Risk
Oxygen Devices, Including High-Flow Nasal Cannula
Before the COVID-19 pandemic, Hui and colleagues published a study on the exhaled gas dispersion during HFNC treatment utilizing smoke light detection on a human patient simulator.24 These investigators found a significant increment in the smoke dispersion distance as the gas flow increased from 10 L/min to 60 L/min. Moreover, they noticed a considerable increase in smoke dispersion distance (up to 620 mm) when the connection between nasal prongs and the simulator nares was loose.24 These findings caused concerns about utilizing HFNC to treat patients with COVID-19. A comparison of the smoke dispersion distances reported by the same researchers using the same technology on different types of oxygen devices found similar dispersion distances with HFNC and conventional oxygen devices, including a standard low-flow nasal cannula and oxygen masks.23,31
Takazono and co-workers used laser light scattering technology and observed no significant differences in dispersion distances between HFNC and other oxygen devices in 3 healthy volunteers,25 regardless of breathing activities such as breathing at rest, coughing, or talking (Table 2). More importantly, compared to coughing, a negligible dispersion was observed with HFNC at a flow of 60 L/min.25 Interestingly, Dellweg and colleagues evaluated exhaled emission during HFNC at 60 L/min flow on a single healthy subject using a schlieren optical system.26 The investigators reported that HFNC increased exhaled air dispersion distance compared with baseline. The maximal dispersal distances while normally breathing room air, HFNC at flows of 20 L/min, 40 L/min, and 60 L/min were 0.99 m, 2.18 m, 2.92 m, and 4.10 m, respectively.26 The inconsistent findings between investigators are probably due to the different methodologies employed; schlieren imaging detects exhaled emission through density differences in the room air, whereas smoke light detection mixes smoke (< 1 µm) with the gas flow to enable measurements. Thus, it is not surprising to see longer dispersion distances with higher HFNC flows in the schlieren imaging measurements.
Investigations of Aerosol Transmission Risk With Different Oxygen Devices, Including High-Flow Nasal Cannula and Noninvasive Ventilation
To determine whether HFNC is an AGP, several researchers compared the concentrations of aerosol particles generated by HFNC with other oxygen devices and different breathing activities. Seven studies among healthy volunteers and 3 studies among patients with COVID-19 found no significant differences in aerosol particle concentrations between HFNC and conventional oxygen devices or breathing room air (Table 2).25,32-41 More importantly, 6 studies reported higher aerosol particle concentrations with coughing than HFNC at 50–60 L/min.25,32,33,36,38,40 Jermy et al reported that the quantity of aerosols generated by a minute of coughing would take the subjects 86 h to generate while breathing normally with HFNC.38 Interestingly, HFNC was reported to reduce the aerosol particle concentrations associated with coughing, sneezing, and snorting.32,38 Therefore, based on the CDC definition, it can be concluded that HFNC is not an AGP.
All the studies mentioned above indirectly assessed the transmission risk with oxygen devices. The most direct and definitive way to evaluate the real risk of transmission is to detect viable viruses in the exhaled aerosol during the treatments. Before the COVID-19 pandemic, Leung and colleagues conducted a randomized crossover trial comparing environmental contamination during HFNC at 60 L/min and oxygen mask at 8.6 ± 2.2 L/min among 19 subjects with pneumonia. Room air sampling plates were placed at distances of 0.4 m and 1.5 m from the subjects and no significant differences in bacterial counts were found between oxygen modalities.42 Two groups of researchers (Lebreil et al43 and Roca et al44) measured the SARS-CoV-2 RNA copies on surfaces and in the room air of subjects with COVID-19 receiving HFNC compared to subjects receiving invasive mechanical ventilation. Invasive mechanical ventilation was considered to present the lowest possibility of environmental contamination because intubated subjects were breathing via the ventilator and their exhaled gas was filtered. Both studies were conducted in single-bed, negative-pressure ICU rooms. There were no significant differences in SARS-CoV-2 RNA copies on surfaces and room air between subjects with COVID-19 treated by HFNC versus invasive ventilation.43,44 Notably, no viable virus was detected by cell culture assays, which agreed with Li and colleagues’ findings among 9 subjects with COVID-19 treated by HFNC.41
Beyond these in vivo and in vitro studies, Westafer and colleagues evaluated COVID-19 infection rates among clinical and non–clinical staff in their emergency department before and after implementing a respiratory protocol that involved using HFNC to treat patients with COVID-19. They found that the infection rates were similar before and after protocol implementation and between clinical and non–clinical staff,45 suggesting that the use of HFNC in patients with COVID-19 did not increase the risk of acquiring infection among the staff.
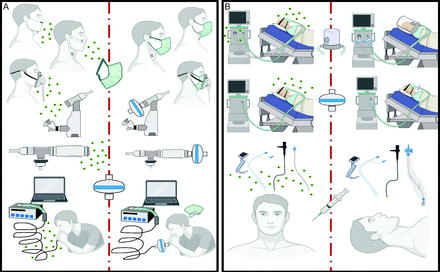
In addition to evaluating aerosol transmission risk during oxygen therapy, investigators also explored mitigation strategies to reduce the aerosol particle concentrations or dispersion distances to confine bioaerosol spread and prevent environmental contamination (Table 3). In subjects with COVID-19, Li and co-workers reported reduced aerosol particle concentrations (0.5–5 µm diameter) after placing a procedural mask over HFNC, particularly at a distance of 1 foot from the subjects.41 Similarly, Takazono et al reported lower aerosol particle concentrations when a procedural mask was placed over the healthy volunteer’s face, regardless of the oxygen devices employed (nasal cannula at 5 L/min or HFNC at 60 L/min) or breathing activities (resting breathing, speaking, or coughing).25 In addition, coughing generates high concentrations of aerosol particles that contain viable viruses, and cough in patients is unpredictable. Thus, wearing the procedural mask for patients with COVID-19 while health care workers are present could be a pragmatic and efficient method to reduce virus transmission. To address concerns that wearing the procedural mask over the subject’s face might cause rebreathing and increase CO2 concentration, Montiel and colleagues placed a procedural mask over HFNC for patients with COVID-19 and reported a slight but not clinically important increment in PaCO2 accompanied by a significant improvement of oxygenation (PaO2/FIO2).46 When oxygen interfaces such as simple masks, Venturi masks, or non-rebreather masks (NRMs) are utilized, subjects could wear procedural masks underneath their oxygen masks (Fig. 1).
Mitigation strategies for different respiratory care procedures. Aerosol particle concentrations can be reduced during oxygen therapy by placing a procedural mask over the nasal cannula or underneath the oxygen mask (A top), by placing a filter at the end of the mouthpiece during nebulization to reduce fugitive aerosol emissions (A middle), or by placing a filter at the mouthpiece during spirometry. Patients need to wear procedural masks immediately after removing their mouth from the mouthpiece (A bottom). During noninvasive ventilation, placing filters at the inlet and outlet of a dual-limb ventilator (B top), using a helmet rather than a face mask (B middle), or placing a filter between the face mask and exhalation port for a single-limb ventilator can reduce aerosol particle concentrations. During intubation or flexible bronchoscopy examinations, aerosol particle concentrations are lower when procedural sedation is used (represented by syringe and needle). Open suctioning should be avoided (B bottom).
Effectiveness of Mitigation Devices During High-Flow Nasal Cannula Treatment and Noninvasive Ventilation
In summary, the risk of viral transmission is relatively low when oxygen therapy, including HFNC, is employed to treat patients with COVID-19. When HFNC is utilized, ensuring a tight connection between nasal prongs and the patient’s nares can reduce the distance to which aerosol is dispersed. Compared to oxygen therapy, vigorous breathing activities such as coughing, deep breathing, and speaking generate considerably higher amounts of aerosols containing viable viruses. Covering the mouth during respiratory activities is highly recommended as a mitigation strategy to reduce viral transmission. Regardless of the type of oxygen device employed, placing a procedural mask over the patient’s face is a rational and effective measure to limit the transmission of bioaerosols.
Noninvasive Ventilation
Several investigators reported no significant differences in aerosol particle concentrations (measured by aerosol particle sizers) generated by NIV or CPAP compared to HFNC (Table 2).32,33,36,40 However, McGain et al found higher aerosol particle concentrations with NIV than HFNC in their healthy volunteers.34 The extent of a leak in and around the mask could explain the differences in the findings. In the 4 studies mentioned above, non-vented masks were utilized with good fit and seal,32,33,36,40 whereas McGain et al intentionally created a leak via the mask by inserting a nasogastric tube to simulate the “worst-case scenario for aerosol generation.”34 Similarly, Simonds and colleagues reported that the concentration of large particles in the environment increased among subjects with chronic lung diseases and coryza symptoms while using a vented mask during NIV.47 In contrast, no significant changes in particle concentrations from baseline were found when NIV was used with the modified circuit that combined a non-vented mask with an exhalation filter.47 Likewise, Hui et al compared smoke dispersion among different types of NIV interfaces; they observed low concentration smoke with a non-vented mask in contrast to high concentration smoke with the vented mask at a close distance.48 Moreover, by employing a helmet with an air cushion around the manikin’s neck, they reduced the leakage of smoke to negligible levels.49
Avari and colleagues nebulized a bacteriophage via a manikin’s trachea and measured the bacteriophage concentrations at 6 different distances with different respiratory support devices.50 They reported higher bacteriophage concentrations with HFNC, NIV, nasal cannula, and NRMs than with invasive ventilation and helmet combined with PEEP valve at the intubation position.50 Winslow and colleagues measured air and surface environmental contamination with SARS-CoV-2 virus in the room of subjects who received CPAP or HFNC treatment.51 Compared to those subjects who used conventional oxygen therapy, they did not find any significant differences in the groups receiving CPAP or HFNC treatments.51 Likewise, Thuresson et al found that the number of positive air samples with SARS-CoV-2 detection was similar in subjects treated with versus without respiratory support (HFNC or NIV).52 Thus, we may conclude that neither NIV nor CPAP is an AGP. Vented masks should be avoided for patients with airborne disease; if tolerated, a helmet with an air cushion around the neck may be preferred. A good fitting properly sized mask with an appropriate seal is needed when a face mask is utilized. Placing an expiratory filter distal to the exhalation valve with a non-vented mask reduces aerosol particle concentrations (Fig. 1), especially in patients who are coughing frequently.
Regarding the mode and settings, Hui et al reported greater smoke dispersion distances when the driving pressure was increased with both vented and non-vented masks.48 However, no significant differences in aerosol particle concentrations between CPAP and NIV among healthy volunteers were found in 3 studies nor between different settings.32,36,53 Clinically, ventilator settings should be set or adjusted based on patient needs. Additionally, Wilson did not find significant differences in aerosol particle concentrations between single-limb ventilators and dual-limb ventilators.36
Aerosol Therapy
In the early phases of the pandemic, there were serious concerns that aerosol therapy, especially nebulization, was an AGP and its use could enhance viral transmission and pose a risk to health care workers.54 Two clinical studies,47,55 one healthy volunteer study30 and one in vitro study,56 reported that aerosol particle concentrations, particularly in small aerosol particles (≤ 5 µm), in the ambient air increased by ∼100 times with nebulization (Table 4). Using the smoke light detection technology, Hui et al also reported greater smoke dispersion distances with a small-volume nebulizer (SVN) driven by 6 L/min air than with an oxygen mask at the same gas flow, especially in the simulated scenario of severe lung injury.57 Additionally, Tang et al placed a virus tracer in a Collison nebulizer and placed the nebulizer at the manikin’s trachea level to produce an exhaled virus.58 They employed an SVN with sterile water to provide nebulization treatment for the manikin and measured the virus copies in air samples at various positions surrounding the manikin. They reported positive results in air samples and concluded that nebulization was a potential source for airborne transmission. However, they did not have a control group (without SVN treatment) for comparison, thus casting doubt on their conclusion. Finally, in the systemic review and meta-analysis conducted by Chan et al, nebulization was reported to have increased the odds of health care workers being infected by the SARS-CoV-1 or SARS-CoV-2 virus.59 However, only 3 studies were included in this analysis; 2 of them reported zero infection rate in both groups, whereas only one small study (N = 32) found a higher infection rate in health care workers who provided nebulization treatment for patients with SARS in 2003 compared to those who did not provide nebulization treatment.60 The medical services have evolved since the initial SARS outbreak when SVN was the primary method for aerosol administration; thus, the relevance of this finding in the context of COVID-19 is limited. Despite the questionable evidence above, nebulization was still listed as an AGP in several guidelines or consensus statements, and nebulizer use was severely limited during the early stages of the pandemic.
Investigations of Aerosol Transmission Risk by Nebulization
To clarify the transmission risk of nebulization, we need to understand the differences between medical aerosols, fugitive aerosols, and bioaerosols. Medical aerosols are the particles generated by a nebulizer or inhaler to deliver medication to the airways and lungs of patients; if the nebulizer and the medication solution are not contaminated, aerosol particles produced should not contain a virus.61 Only part of the medical aerosols would be inhaled and deposited in the subject’s airway; most of the particles are released or exhaled into the environment, the so-called fugitive aerosol.30,56 In contrast, bioaerosols are particles that contain microorganisms, such as the aerosol particles exhaled by an infected individual or the aerosols generated by a contaminated nebulizer. Inhaled medical aerosols either deposit in the airways or are exhaled. There is no known mechanism by which inhaled medical aerosols would be infected by viral-contaminated secretions in the lung or by bioaerosols emitted from patients.61 Notably, inhaled medications might alter the respiratory tract lining fluid and associated airway surface tension, surfactant metabolism, airway diameter, and closing volume of the lung. These physical changes at the airway level could potentially alter the number of exhaled particles (with virus). Thus, microbiological studies in patients with various respiratory diseases treated with different aerosolized medications are needed to validate this assumption. Nevertheless, whereas the use of a nebulizer is expected to increase aerosol particle concentrations in the ambient air, as long as the nebulizer is not contaminated these aerosol particles should not be sources of viral transmission.
Understanding the probability of contamination in different nebulizers plays a crucial role in choosing the nebulizer for patients with airborne diseases. SVNs, like jet or ultrasonic nebulizers, that generate aerosol in the same chamber as the medication reservoir and in which emitted aerosol exits directly into the connection to the patient interface have the highest risk. The nebulizer cup is directly connected to the subject’s mouth via a T-piece, and the patient’s saliva could enter the nebulizer cup. Particularly, when SVN is in repeated use, it can be easily contaminated by environmental sources during the cleaning and storing process, as it is an open system.62,63 Once the nebulizer cup is contaminated, the aerosol generated by the nebulizer would be contaminated with the virus and technically becomes bioaerosol. In contrast, the vibrating mesh nebulizer (VMN) has a medication reservoir that is closed to the ambient air during use, with a mesh separating the medication reservoir from the pathway of emitted aerosol to the patient interface, making contamination from secretions, condensate, or patient-emitted bioaerosols unlikely.61,64 During HFNC and invasive ventilation treatment, the nebulizer, especially a VMN, is recommended to be placed at the inlet of the humidifier, further reducing the risk of retrograde contamination from the patient’s secretions or bioaerosol.
The concentrations of fugitive aerosols generated by nebulizers vary by their design, delivery circuit, and patient interface. Nebulizer design can impact the proportion of medication emitted as an aerosol, whereas the use of operating gas to generate aerosol and carry that aerosol toward the patient contributes to the distribution of particles. For example, the SVN commonly has a residual drug volume of 0.8–1.2 mL, generating less aerosol than the VMN with < 0.1 mL residual volume. However, the SVN operating gas flow (6–10 L/min) disperses emitted aerosol more than the VMN, which requires no gas to operate.61 Both in vitro and in vivo studies found that the use of SVN with an aerosol mask produced the highest fugitive aerosol concentrations, followed by SVN with mouthpiece, VMN with aerosol mask, and VMN with mouthpiece (Table 4).30,56 Notably, placing VMN in line with HFNC generates lower fugitive aerosol concentrations than VMN with mouthpiece.65
Several mitigation strategies are available for different nebulizers with various interfaces (Table 4 and Fig. 1). Placing a face tent scavenger with applied negative pressure over a conventional aerosol face mask is equally effective as a filter face mask that incorporates filters at the exhalation ports in reducing fugitive aerosol concentrations for both SVN and VMN.30 When the filter mask is used, it needs to firmly fit the subject’s face to avoid aerosol leakage. Adding an expiratory filter to the mouthpiece during nebulizer use also reduces fugitive aerosol concentrations. However, patients can still exhale via their nose or via the mouth if the mouthpiece is not tightly sealed. Notably, they may remove the mouthpiece during talking or coughing. Thus, when coughing occurs during nebulization, the mouthpiece should be removed from the mouth, nebulization paused, and cough should be covered to avoid dispersion of patient-generated bioaerosols.61 Moreover, patients should be encouraged to breathe via their mouth and seal the mouthpiece tightly with their lips. For aerosol delivery via HFNC, placing a procedural mask over the patient’s face can reduce fugitive aerosol concentrations, particularly bioaerosols during coughing.
In summary, considering the fugitive aerosol concentrations and the contamination possibilities of different nebulizers with various interfaces, VMN is preferred over SVN for patients with airborne diseases. During aerosol delivery via HFNC, it is recommended for the subjects to wear a procedural mask. When a nebulizer is used with an aerosol face mask for patients with airborne diseases, adding a face tent scavenger with negative pressure or using a filter face mask is suggested, particularly to reduce patient-generated bioaerosols. A mouthpiece should be avoided for frequently coughing patients. Repeated use of SVN should be preferably avoided for patients with airborne diseases. Regardless of the aerosol device employed, clinicians should be aware that patients may cough at any time during aerosol therapy, and they should be instructed to cover their nose and mouth during coughing. If possible, it is always preferred to deliver aerosol treatment in a single room with negative pressure, and clinicians should use appropriate personal protective equipment (PPE), and the number of people inside the patient room should be minimized during and a few minutes after nebulization. Still, it should be acknowledged that the in vivo evidence, especially microbiological evidence linking nebulizer use to the spread of infection, is still lacking, and future studies with microbiological evaluations are warranted to verify if nebulizer treatments during a pandemic are associated with an increased risk for health care workers.
Cough-Precipitating Procedures: Intubation, Extubation, Bronchoscopy Examination, and Open Suctioning
Coughing and sneezing are associated with an increased generation of bioaerosols that contain microorganisms, resulting in higher transmission risk.12 Therefore, medical procedures that precipitate or provoke coughing and sneezing, such as endotracheal intubation, bronchoscopy examination, sputum induction, nasopharyngeal suctioning, and open suctioning for patients with an artificial airway, should be highlighted as AGPs.12,66,67 Several studies have been conducted to examine aerosol particle concentrations and transmission risk during these procedures, and mitigation strategies have been recommended to reduce the risk of transmitting infection.
In the systematic review and meta-analysis conducted by Chan et al, endotracheal intubation was associated with a high odds ratio (6.7) of health care workers contracting SARS-CoV-1 or SARS-CoV-2.59 However, among the 8 cohort studies included in the meta-analysis, 4 were COVID-19 related, but 3 reported zero infection in both health care worker groups that performed intubation and those who did not perform intubation; only one study during the very early stage of the pandemic reported a higher infection rate among health care workers who performed intubation compared to those who did not.68 In that study, only 2 patients with COVID-19 were included; and 6 of 7 health care workers did not wear a mask in the intubation process, whereas only one wore a procedural mask.68 During the intubation for patients with COVID-19, not wearing a mask or wearing a procedural mask is not a standard practice in most high- and middle-resource settings. Thus, Chan et al findings are not relevant to guide current or future practice in those settings.59
Doggette et al measured aerosol particle concentrations during intubation for 16 pigs in a negative-pressure room, and no manual ventilation was provided before intubation. They did not find any significant increment in aerosol particle concentrations compared to baseline (Table 5).69 Brown et al conducted real-time monitoring to quantify aerosol particle concentrations during 19 intubations and 14 extubations in an ultraclean operation theater. The mean aerosol particle concentrations detected in a 5-min period during anesthetic induction and intubation were similar to the background concentrations when no one was in the theater; in contrast, volitional coughs generated 500-fold higher and extubation 15-fold greater aerosol particle concentrations than intubation.70 Similarly, Dhillon and colleagues reported a spike in aerosol particle concentrations during cough.71 Interestingly, both Dhillon et al71 and Reddy et al72 did not find any significant differences in aerosol particle concentrations between intubation and extubation. The differences in these findings might be due to the differences in reporting sizes of aerosol particles assessed by different devices and different room conditions, which included room size, air exchange frequency, the use of positive pressure, etc. Additionally, all the studies were conducted in a very controlled operating room,69-73 which is different from intubating a patient with high minute ventilation, frequent coughing, and copious secretions. Notably, Dhillon et al found that peak aerosol particle concentrations during manual ventilation via a mask and resuscitator, especially after anesthetic induction, were 200–300-fold higher than background concentrations, 71 but such increases were not reported by Brown et al.70 How the manual ventilation was delivered (size of delivered breath, frequency, inspiratory time, etc) and whether a filter was utilized in both studies were not specified. Regardless, when a filter was placed between the mask and the resuscitator, a reduction in aerosol dispersion distance was observed.73
Investigations of Aerosol Transmission Risk Associated With Intubation, Extubation, Bronchoscopy Examination, and Tracheostomy Care
An intubation box designed to reduce the direct aerosol path from a patient to the surrounding environment was utilized during the early stages of the pandemic to mitigate the transmission risk of intubation.74,75 However, shortly after its clinical application, several investigators reported that the use of the intubation box was associated with more intubation attempts, increased intubation time, and more breaches of PPE.74,75 It should be noted that patients usually receive sedation and paralytics before intubation, and cough or even spontaneous breathing is suppressed. Although the airway is open, once the endotracheal tube is inserted, the tube will be immediately connected to a ventilator. The time of exposure to the subject’s lower airway is very short; thus, the risk of generating aerosols, especially bioaerosols, would be low. In contrast, during extubation, subjects’ spontaneous breathing returns; they are not sedated, and they may have a higher incidence of cough, leading to increased aerosol particle concentrations in the environment.76 Nevertheless, when the patients are ready to be extubated, especially those who are intubated due to COVID-19, they may have largely recovered from COVID-19, and the viral load in their exhaled gas may be negligible. Thus, the infection risk posed by extubation might remain low. However, appropriate precautions must be taken during these procedures to mitigate the risk of viral transmission to health care workers.
During elective flexible bronchoscopy examinations with procedural sedation for COVID-19–negative patients, no significant increment of aerosol particle concentrations was observed in 2 clinical studies, except that higher aerosol particle concentrations were found when lidocaine was atomized (Table 5).69,72 Furthermore, Doggette et al noted that aerosol particle concentrations varied among patients with different etiologies or procedures, such as suctioning or bronchoalveolar lavage.69 Future studies are needed to investigate the aerosol particle concentrations emitted by different procedures and, more importantly, the infectivity of the aerosols generated during those procedures for patients with airborne diseases. Additionally, Reddy et al found higher aerosol particle concentrations during rigid bronchoscopy with jet ventilation under general anesthesia than flexible bronchoscopy examination.72 However, they did not measure the amount of aerosols generated by jet ventilation alone; thus, the contribution of rigid bronchoscopy to the aerosol particle increment was not determined.
Tracheostomy care, especially open suctioning for patients no longer receiving mechanical ventilation, is concerning as the lower airway of these patients is directly open to the ambient air. In an in vitro study, a nebulizer was placed at manikin’s trachea to simulate exhaled aerosols.77 Compared to uncovered tracheostomy, a simple cover such as a cotton mask reduced the aerosol particle concentrations.77 The combination of a procedural mask with a heat-moisture exchanger (HME) was found to be the most effective (Table 5), but the authors did not clarify the position of the procedural mask and the HME; presumably, the procedural mask was placed over the HME. The practical value of this placement is questionable, as the procedural mask might be easily misplaced by subject movement or coughing. Thus, a filter HME might be a better option. More importantly, wearing a procedural mask over the face for tracheostomy patients with full or partial cuff deflation is as critical as placing a filter HME on the tracheostomy tube. In stable patients who coughed very infrequently, Li et al found no significant differences in aerosol particle concentrations with or without mitigation devices, including HME, among 12 tracheostomy patients with cuffless tubes or cuff deflated.78 An explanation for the findings of their study could be that none of the patients wore a procedural mask over their face, and aerosol particles exhaled via their upper airways could still contribute to the aerosol particle concentrations in the ambient air.78
Open suctioning is of concern for patients with artificial airways, especially those receiving invasive ventilation. The direct stimulation of the airway with a suction catheter precipitates coughing. Bioaerosols from the lower airways can be dispersed mainly to the surrounding environment by the high-velocity exhaled gas during open suctioning; thus, open suctioning enhances the risk of transmitting infection when performed in patients with airborne diseases receiving invasive ventilation. Closed suction systems would be preferred for such patients. For patients with tracheostomy, especially those receiving tracheostomy due to long-term mechanical ventilation after COVID-19 infection, many have recovered from COVID-19 by the time they are weaned off from the ventilator. As such, the infectivity of their exhaled bioaerosols could be low. In contrast, for patients who had tracheostomy prior to COVID-19 infection, placing a filter HME for them is crucial if they do not require ventilator support. If active humidification is needed, connecting a T-piece suctioning catheter with a humidifier or a large-volume nebulizer and the other end to a filter and simultaneously wearing a procedural mask over the patient’s face might be a rational choice.
Pulmonary Function Testing
Pulmonary function tests are valuable assessments that provide essential information for diagnosing, monitoring progress, and managing respiratory diseases.79,80 Concerns have arisen regarding the transmission risk during deep-breathing maneuvers and activities performed during testing that could generate aerosol particles.81 Two studies in healthy volunteers and 3 clinical studies found significantly increased aerosol particle concentrations during spirometry tests (Table 6).81-84 However, those particle concentrations are lower than coughing. Adding a viral filter to the mouthpiece or to a tightly fitting mask can reduce aerosol particle concentrations without significantly influencing the pulmonary function testing variables.81
Investigations of Aerosol Transmission Risk Associated With Pulmonary Function Testing and Cardiopulmonary Exercise Testing*
During cardiopulmonary exercise, aerosol particle concentrations increased significantly when the subject’s heart rate reached ≥ 50% of the predicted heart rate reserve among healthy volunteers who were not wearing a mask (Table 6). Higher-intensity exercises generated greater aerosol particle concentrations.85 Similarly, when healthy volunteers wore procedural masks during cardiopulmonary exercise, light-to-moderate exercise did not generate higher aerosol particle concentrations, whereas hard training did.86 Even with donning of procedural masks during cardiopulmonary exercise, aerosol particle concentrations were significantly increased when subjects exercised at a somewhat hard level with heart rates reaching two thirds of predicted maximum heart rates. The increased concentrations were associated with the increased number of participants in exercise sessions.87 As such, a more efficient mitigation strategy is warranted.86-88 Garzona-Navas et al utilized a portable high-efficiency particulate air filter with a fume hood in their healthy volunteer study. They found these devices significantly reduced aerosol particle concentrations during exercise, especially small particles at a size of ≤ 1 µm.88
Notably, cough is commonly provoked by deep breathing or exercise; clinicians should always don PPE during pulmonary function tests or cardiopulmonary exercise testing, especially those who are in the close vicinity of such patients. Additionally, the room air needs to be cleaned after the test.82 The safe interval between tests depends on the room size, air exchange frequency, and concentration of aerosol particles generated by previous breathing activities.
Summary
Transmission risks of respiratory care procedures rely on the production of bioaerosol particles by the infected subjects, which carry the microorganisms. Coughing generates significant amounts of bioaerosols; thus, any procedures, such as nasal-pharyngeal suctioning, open suctioning for patients with artificial airways, and bronchoscopy examination for non-intubated patients, that provoke cough in patients should be considered as AGPs with high-transmission risks. In contrast, treatments that might disperse the exhaled particles to a further distance, such as HFNC or NIV, should be considered as aerosol-dispersing procedures with little to no additional risk of transmitting infection. Even though nebulization generates high quantities of fugitive aerosols in the ambient air, the transmission risk for these medical aerosols remains low if the nebulizer is not contaminated. Aerosol delivery via HFNC or VMN with a mouthpiece or face mask potentially has a lower transmission risk than SVN, due to the lack of dispersion of aerosol by operating gas flow and the low probability of contamination. Placing a procedural mask over HFNC, adding an expiration filter at the end of the mouthpiece, and using a filter face mask or a face tent scavenger can reduce aerosol particle concentrations in the environment. Lastly, noninvasive respiratory support, including HFNC and NIV, is not an AGP, but patients may cough at any moment while using those devices, and mitigation strategies such as wearing a procedural mask over HFNC or adding a filter between the mask and exhalation port during NIV are recommended. Regardless of the procedure types and mitigation strategies, health care workers should always take precautions while taking care of patients with airborne disease and use appropriate PPE during exposure to AGPs or aerosol-dispersing procedures. Whereas many policies or guidelines that hospitals adopted during the early pandemic were based on limited evidence, the increasing body of evidence that has assessed the transmission risk posed by various respiratory care procedures has provided greater perspective. This evidence should be considered by key decision makers to revise their policies and guidelines.
Acknowledgment
We appreciate Dr Danlei Huang for drawing the figure.
Footnotes
- Correspondence: Jie Li PhD RRT RRT-ACCS RRT-NPS FAARC, 600 S Paulina Street, Suite 765, Chicago, IL. E-mail: Jie_Li{at}rush.edu
Dr Li discloses relationships with The Rice Foundation, American Association for Respiratory Care, Fisher & Paykel Healthcare, Aerogen, and Heyer. Dr Li also serves as section editor for Respiratory Care. Dr Fink is chief science officer for Aerogen Pharma Corp. Dr Dhand discloses relationships with GlaxoSmithKline, Boehringer Ingelheim, Mylan, Teva, and AstraZeneca. Ms Alolaiwat has disclosed no conflicts of interest.
Dr Li presented a version of this paper at the New Horizons Symposium: COVID-19 Lessons Learned at AARC Congress 2021 LIVE!, held virtually December 3, 2021.
- Copyright © 2022 by Daedalus Enterprises
References
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.
- 15.
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.
- 28.
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.
- 36.↵
- 37.
- 38.↵
- 39.
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- 64.↵
- 65.↵
- 66.↵
- 67.↵
- 68.↵
- 69.↵
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.↵
- 75.↵
- 76.↵
- 77.↵
- 78.↵
- 79.↵
- 80.↵
- 81.↵
- 82.
- 83.
- 84.↵
- 85.↵
- 86.↵
- 87.↵
- 88.↵