To the Editor:
In a recent issue of Respiratory Care, Mireles-Cabodevila and Kacmarek1 did an excellent job reviewing the pros and cons of airway pressure release ventilation (APRV). The paper and the discussion session raised serious concerns about ARPV, particularly regarding imposed work of breathing and excessive tidal volumes due to high peak transpulmonary pressures. However, neither in the paper (except for a brief reference by Dr Mireles-Cabodevila) nor in the discussion was there an emphasis on what we believe is the most dangerous risk of using APRV as a rescue treatment for severe ARDS: occult atelectrauma.
Although the use of APRV has almost disappeared at our institution (Cleveland Clinic), a few years ago there was a lot of interest in the mode. As a result, we got involved in some research on the topic, producing a few papers and abstracts. What always intrigued us was the frequent justification by supporters for using APRV that “it works,” meaning that oxygenation improved in patients with severe ARDS, yet patients with severe ARDS rarely die of hypoxemia (∼10%); they die of multi-organ failure. Organ failure is linked to the release of inflammatory mediators from the lung in response to mechanical trauma. Hence, focusing on oxygenation as the main goal of APRV at the expense of lung-protective ventilation does not seem like the most rational approach.
Oxygenation problems are usually managed with PEEP. Patients with severe ARDS should have end-expiratory lung volume manipulated by some form of optimal PEEP heuristic. However, the most vehement supporters for APRV recommend setting zero PEEP (ie, Plow = 0 cm H2O but setting Tlow short enough to maintain adequate end-expiratory lung volume by means of auto-PEEP).2 However, reliance on auto-PEEP instead of set PEEP may result in unknown and unstable lung volumes and hence unstable mechanical support of gas exchange (not to mention the uneven distribution of auto-PEEP in the lungs according to the distribution of unequal time constants of lung units, with the sickest units getting the least PEEP).
Furthermore, changes in lung mechanics (eg, the need for suctioning, variable edema, and variable inspiratory effort) make auto-PEEP an unpredictable random variable in an individual patient. We have programmed a high-fidelity physical breathing simulator with actual values for resistance and compliance from patients with severe ARDS ventilated with APRV and demonstrated that with a ventilator connected to the simulator and the actual ventilator settings used (according to published APRV recommendations), there was no auto-PEEP at all (unpublished data). No PEEP in a patient with severe ARDS certainly suggests an increased risk of ventilator-induced lung injury due to atelectrauma.
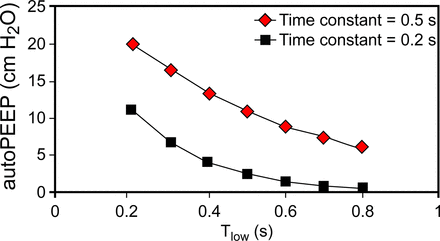
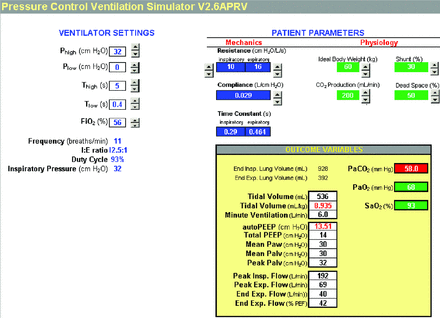
Auto-PEEP can be estimated with mathematical models.3,4 Patients with severe ARDS have a wide range of respiratory system expiratory time constants (average 690 ± 280 ms).5,6 Assuming7 an expiratory time constant of 0.5 s, setting Tlow in the range of 0.2–0.8 s2 results in auto-PEEP ranging from 2 to 20 cm H2O with Phigh set at 30 cm H2O (relative to atmospheric pressure) and Plow = 0 in a passive patient with ARDS (see Fig. 1). Lower Phigh values (ie, <30 cm H2O) naturally result in lower ranges of auto-PEEP. Auto-PEEP for this figure was estimated using the equation, auto-PEEP = (Phigh − Plow) × e−(Tlow/RC), where RC is the expiratory time constant and e is the base of natural logarithms (∼2.72). This equation describes a simple exponential decay of pressure in response to a step change from Phigh to Plow (assumed to be zero in this case) over the period of Tlow. We have created a Microsoft Excel-based simulator based on published equations3 that can be used to understand how ventilator settings during APRV affect lung volumes, flows, and pressures along with estimated PaCO2 and PaO2 for different values of passive lung mechanics (free to download at http://is.gd/G6hD0A), as shown in Figure 2. It is very helpful in understanding the interdependencies among Phigh, Plow, Thigh, Tlow, auto-PEEP, and passive tidal volume (so-called “release volume”). We would argue that the use of such a simulator is the only practical way to gain understanding of APRV, because equivalent experience with real patients could take years and put a lot of people at risk. As we noted in a previous paper,4 APRV is more complex than it appears to be. It requires a lot more knowledge and skill than may be apparent from descriptions in the literature.
Predicted auto-PEEP during airway pressure release ventilation with Phigh = 30 cm H2O and Plow = 0 cm H2O. Expiratory time constant (expressed in s) is the product of resistance × compliance.
Microsoft Excel-based airway pressure release ventilation simulator. Courtesy Mandu Press.
In contrast to expected auto-PEEP levels during APRV, “optimum” PEEP levels recommended by the ARDSNet higher-PEEP table8 for patients requiring FIO2 ≥0.60 range from 20 to 24 cm H2O. Thus, reliance on auto-PEEP during APRV probably results in suboptimal end-expiratory lung volume at least some of the time. Add to this the problem that some ventilators synch the ending of Thigh with spontaneous expiration during Phigh, and you get an unpredictable Tlow despite explicit settings.9,10 On the other hand, triggering the transition from Plow to Phigh using expiratory flow is helpful, but it does not avoid the interdependence of auto-PEEP and tidal volume.4,11
Here is the main point of our letter: The risk of atelectrauma (a distant and hidden effect) is often discounted by clinicians in favor of the benefit of improved oxygenation (immediate and obvious effect). This is an example of the decision error known as present moment discounting (the tendency for people to have a stronger preference for more immediate payoffs relative to later payoffs).12 Unfortunately, when outcomes of ventilation with APRV are unsatisfactory, they are often rationalized on the basis of poor prior probability of survival due to ARDS (obvious) rather than the potential for suboptimal ventilator management (hidden).
In addition, there has yet to be a thoughtful discussion regarding the impact of APRV on right heart function. In ARDS, acute cor pulmonale occurs in 22–25% of patients and reaches 50% among those with severe presentations.13,14 Of even greater concern is that mortality is substantially greater (67% vs 49%) in those with moderate to severe ARDS who develop acute cor pulmonale,14 How this relates to a discussion of whether APRV is a prudent approach to lung-protective ventilation in ARDS is seen by consideration of the sobering evidence that elevated plateau pressure 27–35 cm H2O) is linked to developing acute cor pulmonale and that an additive effect of elevated plateau pressure and cor pulmonale increases mortality risk.14,15 The deleterious effects of sustained, high intrathoracic pressures producing hemodynamic compromise probably explain the unexpectedly higher mortality in those managed with high-frequency oscillatory ventilation.16,17 Others have also pointed out that the heterogeneous distribution of time constants in ARDS very likely leads to heterogeneous distribution in regional lung volumes that not only may induce/exacerbate regional atelectrauma, as we suggest, but also regional alveolar overdistention.14
In summary, the use of ARPV (with extreme inverse I:E and Plow = 0) as a rescue strategy for ARDS results, at least theoretically, in (1) increased work of breathing, (2) increased risk of volutrauma, (3) increased risk of atelectrauma, and (4) increased risk of cor pulmonale, compared with other pressure control modes. To us, these theoretical risks outweigh the potential improvement in PaO2. If there were no alternative, then the risks might be warranted. But as we have shown,4 you can obtain the same objectives as APRV (ie, the same values for mean airway pressure, end-inspiratory lung volume, “release volume,” and end-expiratory lung volume) using a known set PEEP value and a Tlow set long enough to avoid auto-PEEP. This approach decouples the level of mechanical support from the level of auto-PEEP, making clinical management easier and more predictable.
Perhaps the last word should go to John Downs (the inventor of APRV) and colleagues who commented on the largest study of APRV to date.18 In a letter to the editor in the Journal of Trauma, they said “Many clinicians use APRV as a rescue mode for the treatment of ARDS. No study supports…the use of APRV in that way…”19 In their response to the letter, the authors of the study stated that “We do not believe that APRV should be used as a rescue mode either.”
Footnotes
Mr Chatburn has disclosed relationships with IngMar Medical and DeVilbiss/Drive Medical. The other authors have disclosed no conflicts of interest.
- Copyright © 2016 by Daedalus Enterprises