Abstract
BACKGROUND: Transcutaneous monitors are utilized to monitor a patient's respiratory status. Some patients have similar values when comparing transcutaneous carbon dioxide (PtcCO2) values with blood gas analysis, whereas others show extreme variability. A retrospective review of data was performed to determine how accurately PtcCO2 correlated with CO2 values obtained by arterial blood gas (ABG) or capillary blood gas.
METHODS: To determine whether PtcCO2 values correlated with ABG or capillary blood gas values, subjects' records were retrospectively reviewed. Data collected included the PtcCO2 value at the time of blood gas procurement and the ABG or capillary blood gas PCO2 value. Agreement of pairs of methods (ABG vs PtcCO2 and capillary blood gas vs PtcCO2) was assessed with the Bland-Altman approach with limits of agreement estimated with a mixed model to account for serial measurements per subject.
RESULTS: A total of 912 pairs of ABG/PtcCO2 values on 54 subjects and 307 pairs of capillary blood gas/PtcCO2 values on 34 subjects were analyzed. The PCO2 range for ABG was 24–106 mm Hg, and PtcCO2 values were 27–133 mm Hg. The PCO2 range for capillary blood gas was 29–108 mm Hg, and PtcCO2 values were 30–103 mm Hg. For ABG/PtcCO2 comparisons, the Pearson correlation coefficient was 0.82, 95% CI was 0.80–0.84, and P was <.001. For capillary blood gas/PtcCO2 comparisons, the Pearson correlation coefficient was 0.77, 95% CI was 0.72–0.81, and P was <.001. For ABG/PtcCO2, the estimated difference ± SD was −6.79 ± 7.62 mm Hg, and limits of agreement were −22.03 to 8.45. For capillary blood gas/PtcCO2, the estimated difference ± SD was −1.61 ± 7.64 mm Hg, and limits of agreement were −16.88 to 13.66. The repeatability coefficient was about 30 mm Hg.
CONCLUSIONS: Based on these data, capillary blood gas comparisons showed less variation and a slightly lower correlation with PtcCO2 than did ABG comparisons. After accounting for serial measurements per patient, due to the wide limits of agreement and poor repeatability, the utility of relying on PtcCO2 readings for this purpose is questionable.
Introduction
The use of transcutaneous monitors is standard of care in many NICUs across the country.1 Additionally, blood gas analysis has been recognized as the definitive measurement used to gauge a patient's ventilatory and oxygenation status. Several authors have referred to blood gas analysis as the accepted standard with which all other methods are compared.2–4 However, procurement of a blood gas specimen can result in arterial spasm, pain, infection, distorted values if the specimen is being obtained by arterial puncture, and iatrogenic anemia.3 Many comparative studies have been undertaken between transcutaneous monitors and blood gas analysis. A PubMed search (using the search term “transcutaneous CO2 monitoring”) returned 305 publications. After applying a filter (“infant: birth – 23 mo”), a total of 73 publications were returned. Some studies have shown reasonable agreement between the 2 methods, whereas others have shown a great deal of variance.
The development, description, and principles of operation of transcutaneous monitors have been extensively reviewed elsewhere.2,5 The American Association for Respiratory Care Clinical Practice Guidelines caution that inappropriate treatment, including ventilator manipulation, may result from misinterpretation of falsely elevated or decreased PtcCO2 values.5 A 2016 report, using single measurements, concluded that PtcCO2 values could not replace PaCO2 by arterial blood gas (ABG).6 This study was undertaken to determine whether repeated measurements of ABG PCO2 values on patients were closer in agreement than repeated capillary blood gas PCO2 values when compared with PtcCO2 obtained from a transcutaneous monitor.
QUICK LOOK
Current knowledge
Transcutaneous monitoring has been extensively studied, although randomized controlled trials are limited. Various studies have shown that agreement between PtcCO2 and ABG or capillary blood gas is variable, limiting clinicians' ability to rely on PtcCO2 values for trending purposes only.
What this paper contributes to our knowledge
After accounting for serial measurements per subject, due to the wide limit of agreement and poor repeatability, the utility of relying on PtcCO2 readings is questionable. Clinicians should view transcutaneous monitor values with suspicion and utilize critical thinking before performing interventions.
Methods
This was a non-interventional observational retrospective study including all subjects who had simultaneous blood gas analysis and were on a transcutaneous monitor. The University of Arkansas for Medical Sciences institutional review board determined the study protocol did not meet the definition of human subject research and was exempt from review. A list of all patients who received a charge for transcutaneous monitor supplies was requested and obtained from the Patient Accounts Department spanning the time period from July 1, 2014 through January 31, 2016. Patient records were reviewed and yielded a total of 1,235 specimen comparisons on 88 subjects (Fig. 1). Of this total, 12 venous blood gas specimens were excluded, as the original concept was to compare ABG and capillary blood gas with PtcCO2 values. Two ABG values were excluded because the results were above the reportable range (>128 mm Hg) on the blood gas analyzers (GEM 4000, Instrumentation Laboratory, Bedford, Massachusetts) that were used during the study. Two ABG values were excluded because the results were below the reportable range (<17 mm Hg). This left a total of 1,219 specimen comparisons with PtcCO2 on 88 subjects. This was further divided into 912 ABG/PtcCO2 comparisons on 54 subjects and 307 capillary blood gas/PtcCO2 comparisons on 34 subjects. All PtcCO2 values were obtained from the transcutaneous monitor TOSCA Monitor (Radiometer America, Brea, California).
Flow chart. ABG = arterial blood gas; CBG = capillary blood gas.
Data points were collected and recorded based on the routine practice in the NICU. Once the physician ordered the ABG or capillary blood gas, nursing staff entered the order into the electronic medical record. Upon completion of the order entry, a requisition was printed. The nurse performed the blood gas collection, and the PtcCO2 value was noted during specimen acquisition and recorded on the requisition. The requisition and the specimen were sent to the laboratory via the pneumatic tube system. The specimen was analyzed, and the PtcCO2 value was manually recorded by laboratory personnel and noted in the results for comparative purposes. The results were released and immediately available in the medical record. Omissions or inaccurate PtcCO2 values would be corrected as necessary upon receipt of the results by the ordering nurse. According to department policy, the transcutaneous monitor temperature was set to 40°C, and routine site changes occurred 4 times a day. Calibration of the transcutaneous monitor devices also occurred every 6 h during site changes. Sensors were re-membraned at a maximum of every 14 d or sooner, as indicated by the monitor.
Statistical Analysis
Agreement of pairs of methods (ABG/PtcCO2 and capillary blood gas/PtcCO2) was assessed with the Bland-Altman approach7 with limits of agreement estimated with a mixed model8 (one for each pair) to account for linked repeated measurements per subject. Repeated measurements were linked as pairs of methods (ABG/PtcCO2 and capillary blood gas/PtcCO2) by being taken in parallel; thus, the resulting values shared a common environment (in this case time). All analyses were performed in SAS 9.4 (SAS Institute, Cary, North Carolina). P < .05 was considered statistically significant.
Results
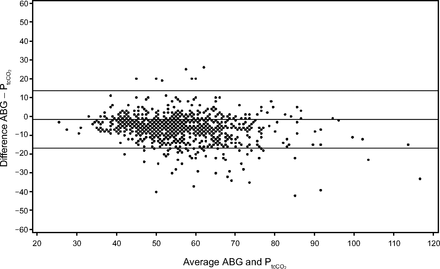
A total of 912 pairs of values on 54 subjects for ABG/PtcCO2, and 307 pairs of values on 34 subjects for capillary blood gas/PtcCO2 were analyzed. The PCO2 range for ABG was 24–106 mm Hg, and the range for PtcCO2 was 27–133 mm Hg. The PCO2 range for capillary blood gas was 29–108 mm Hg, and the range for PtcCO2 was 30–103 mm Hg (Table 1). For the ABG/PtcCO2 pair, the Pearson correlation coefficient was 0.82, 95% CI was 0.80–0.84, and P was <.001, and for the capillary blood gas/PtcCO2 pair, the Pearson correlation coefficient was 0.77, 95% CI was 0.72–0.81, and P was <.001 (Table 2). From mixed models accounting for linked repeated measurements (one for each pair), for the pair ABG/PtcCO2, the estimated difference ± SD was −6.79 ± 7.62 mm Hg, and for the pair capillary blood gas/PtcCO2, the estimated difference ± SD was −1.61 ± 7.64 mm Hg (Table 3). Figure 2 shows the Bland-Altman plot for ABG versus PtcCO2 with a 95% prediction interval (limits of agreement). The limits of agreement are wide and indicate that at any level of PtcCO2, the ABG value may be up to about 15 units above or below the true PtcCO2. From the plot, there is an apparent slight inverse linear trend of difference with average (the expectation is no trend of differences with the average). Figure 3 shows the Bland-Altman plot for capillary blood gas versus PtcCO2 with 95% prediction interval (limits of agreement). The limits of agreement are wide and indicate that at any level of PtcCO2, the capillary blood gas value may be up to about 15 units above or below the true PtcCO2. From the plot, there is an apparent slight inverse linear trend of difference with average. From the same mixed model for each pair, the repeatability coefficients for methods in each pair are shown in Table 4 (note that there are 2 estimates of repeatability for PtcCO2, because PtcCO2 appears in both pairs). For example, the ABG method measured on the same patient under identical physiological/clinical circumstances as patients in the sample may yield readings that differ by up to 28.77 units, whereas the capillary blood gas method may yield readings that differ by up to 29.63 units.
Range of Values Noted for the Comparative Groups
Pearson Correlation Coefficient for Each Pair of Methods With Corresponding 95% CI and P
Estimated Difference, SD of Differences From a Mixed Model to Account for Repeated Measurements on the Same Patient, and Lower and Upper Limits of Agreement
Bland-Altman plot for arterial blood gas (ABG) versus PtcCO2 with 95% prediction interval.
Bland-Altman plot for capillary blood gas (CBG) versus PtcCO2 with 95% prediction interval.
Repeatability Coefficients for Methods in Each Pair
Discussion
As noted earlier, transcutaneous monitor comparisons have been studied since the devices became approved for clinical use. Many studies have compared transcutaneous monitors with blood gases, whereas others have compared transcutaneous monitors with end-tidal CO2 measurements. The overall consensus is that transcutaneous monitors can be used to trend the patient's status but should not be relied on to make significant changes to patient care without corroborating information, preferably from blood gas analysis. This may lead to significant challenges for the health-care team. For example, when looking at the individual subject data in this study, there appeared to be at least one set of the comparative data that had values that were >± 15 mm Hg different between the blood gas and transcutaneous monitor reading (see supplementary materials at http://www.rcjournal.com). More specifically, subject number 19 had 20 comparisons recorded (Table 5). The mean difference between the ABG and transcutaneous monitor reading was −5.5 mm Hg (range −22 to 1) and overall seemed to be a reliable trend. However, there were 2 readings with differences of −22 and −20 mm Hg. If those values were removed, the mean difference would have dropped to −3.8 mm Hg (range −8 to 1), and the data would have been more reliable as an indication of the subject's clinical state.
Data From Subject 19 Showing Mean Differences With and Without the 2 Extreme Values
This point can be further analyzed using the Pearson correlation coefficient. The magnitude of the Pearson correlation coefficient determines the strength of the correlation. Although there are no hard and fast rules for assigning strength of association to particular values, Evans9 provides the following guidelines where the strength of the correlation can be described verbally for the absolute value of r: r = 0–0.19, very weak; r = 0.20–0.39, weak; r = 0.40–0.59, moderate; r = 0.60–0.79, strong; r = 0.80–1 very strong.
When applying the above guidelines by Evans to the subject's data cited above, the Pearson correlation coefficient for the entire data set yields r = 0.59, indicating only moderate positive correlation. If the 2 extreme value differences (−22 and −20 mm Hg) are removed, then r = .89, indicating a very strong positive correlation. Therein lies the problem. In a clinical situation when decisions need to be made quickly and interventions performed rapidly to address a problem, does one treat the patient without question? When looking at the transcutaneous monitor reading, it may have been a reliable indicator of the subject's ventilatory state 90% of the time, but the 2 significant outliers cause practitioners to question all of the readings. Further, when applying the above guidelines to all ABG/PtcCO2 comparisons, the Pearson correlation coefficient of r = 0.82 indicates a very strong positive correlation, whereas the capillary blood gas/PtcCO2 comparisons yielded a Pearson correlation coefficient of r = 0.77, which indicates a strong positive correlation.
In the population studied, for the ABG/PtcCO2 group, the estimated difference ± SD was −6.79 ± 7.62 mm Hg, and for the capillary blood gas/PtcCO2 group, the estimated difference ± SD was −1.61 ± 7.64 mm Hg. This indicates that capillary blood gas readings were closer in agreement to the actual CO2 values obtained by the transcutaneous monitor. This agrees with the observations by Mukhopadhyay et al,10 who noted that the transcutaneous monitor senses CO2 diffusion from heated capillary beds and conveys the same difference that capillary gases demonstrate from simultaneous arterial gases. Another explanation for the estimated differences could be the fact that ABGs are typically performed via umbilical artery catheter and are continued until the catheter is no longer usable. This is during the acute phase of the neonate's illness when iatrogenic factors come into play, such as the use of vasopressors and more intensive ventilator manipulations. Once the transition to capillary blood gas acquisition begins, the infant may be off vasopressors, and ventilator settings are not as extreme as during the acute phases of the illness. This would allow for the closer agreement noted with the capillary blood gas/PtcCO2 comparisons.
Overall, this study seems to agree with many other studies that have concluded that a transcutaneous monitor should be used as a trending device. Observation of extreme PtcCO2 values should be questioned. As in all instances, clinicians should treat the patient and not the monitor. If an extreme value is noted, the patient should be closely observed and a determination made as to whether the patient “looks” like the PtcCO2 value is accurate. If the patient appears to be in distress, a confirmatory ABG or capillary blood gas should be obtained. If the patient does not appear to be in distress, then recalibration or re-siting of the probe should be considered. One of the initial concerns was that using the transcutaneous monitors actually increased the number of blood gases obtained, thereby increasing cost to the patient. We were unable to quantify this supposition, although the study by Mukhopadhyay et al10 found that the use of transcutaneous monitors did not increase the number of blood gas samples taken and actually reduced the frequency of this intervention.
There are several limitations to this study. First, it was a retrospective, observational study utilizing a specific transcutaneous monitor and blood gas analyzer. Findings could be different with another brand of transcutaneous monitor or analyzer. Second, data were not stratified based on gestational age, type of ventilatory support, or birthweight. Further insights could be gleaned from re-analysis of the data based on these factors. Third, this was a single-institution study, and these results only reflect the experience at this institution and may not be generalizable to other institutions. Fourth, the routine practice in the NICU has been in place for a number of years in recording of PtcCO2 values on the requisition sent to the laboratory during the analysis process. It is possible that errors could have occurred during the transcription process, leading to erroneous data being recorded.
Conclusions
The findings of this study appear to agree with many of the other studies comparing transcutaneous monitor readings with blood gases. Specifically, after accounting for serial measurements per subject, due to the wide limit of agreement and poor repeatability, the utility of relying on PtcCO2 readings for this purpose is questionable. Additional studies are indicated, preferably with randomized controlled trials. Further, manufacturers should endeavor to continue with research and development to find new noninvasive tools that more consistently and accurately measure CO2. Until that point in time, clinicians should view transcutaneous monitor values with suspicion and utilize critical thinking before performing interventions.
Footnotes
- Correspondence: J. Randy Willis MBA RRT RRT-NPS AE-C, Respiratory Care, Slot 303, 1 Children's Way, Little Rock, AR 72202-3591. E-mail: willisjr{at}archildrens.org.
The authors have disclosed no conflicts of interest.
Supplementary material related to this paper is available at http://www.rcjournal.com.
Mr Willis presented a version of this paper as an Editors' Choice abstract at the AARC Congress 2017, held October 4-7, 2017, in Indianapolis, Indiana.
- Copyright © 2018 by Daedalus Enterprises