Abstract
The mixture of oxygen and nitrogen is usually sufficient to achieve the therapeutic objective of supporting adequate gas exchange. Pediatric and neonatal patients have an assortment of physiologic conditions that may require adjunctive inhaled gases to treat the wide variety of diseases seen in this heterogeneous population. Inhaled nitric oxide, helium oxygen mixtures, inhaled anesthetics, hypercarbic mixtures, hypoxic mixtures, inhaled carbon monoxide, and hydrogen sulfide have been used to alter physiology in an attempt to improve patient outcomes. Balancing the therapeutic potential, possible adverse effects, and the complexity of the technical aspects of gas delivery, it is essential that clinicians thoroughly understand the application of medical gas therapy beyond the traditional nitrogen/oxygen mixture.
- inhaled nitric oxide
- heliox
- inhaled anesthetics
- inhaled carbon monoxide
- inhaled carbon dioxide
- hypoxic gas mixtures
- hydrogen sulfide
Introduction
The application of supplemental oxygen is a cornerstone of respiratory care. Unfortunately, a small subset of critically ill patients are refractory to escalating oxygen concentration and/or positive-pressure mechanical ventilation. Adjunctive therapies, including inhaled medical gases beyond oxygen/nitrogen, have been explored with the purpose of supporting adequate gas exchange, improving hemodynamics and minimizing injurious levels of positive-pressure ventilation. As specific therapies continue to evolve, clinicians should have a clear understanding of the physiologic basis and evidence when making decisions regarding any adjunctive therapy.
Many questions remain about the role of these unique gases in the management of neonatal and pediatric patients. Given the additional cost, equipment needs, and technical expertise required for adjunctive inhaled gases, it is paramount that clinicians have a comprehensive understanding of the pros and cons of the potential applications of these gases. The purpose of this paper is to discuss the role of inhaled nitric oxide (INO), heliox, inhaled anesthesia, carbon dioxide, and carbon monoxide in supporting neonatal and pediatric patients.
Inhaled Nitric Oxide
Nitric oxide is a naturally occurring substance found throughout the human body as a neurochemical transmitter. It is in human airways at a concentration of 10–100 parts per billion, in air pollution (smog) at 10–1,000 parts per billion, and in cigarette smoke at 400–1,000 parts per million (ppm).1 Medical dosing of INO gas is generally in the range 1–20 ppm.2 Currently, no definitive evidence suggests any benefit from delivering INO in excess of 20 ppm.
The clinical use of INO has increased remarkably over the past several decades. It was first described in 1987 as “endothelial-derived relaxing factor.”3 Since that time, INO has been the subject of extensive study for the physiologic response in patients with impaired hemodynamics and/or gas exchange. The discovery of INO's role in pulmonary vascular tone led to an abundance of biomedical research from basic science to large randomized clinical trials in patients of all ages, resulting in thousands of publications. In 1992, the journal Science named nitric oxide the “molecule of the year.”4 In 1998, Robert Furchgott, Louis Ignarro, and Ferid Murad jointly received a Nobel prize in medicine and physiology for their discoveries concerning nitric oxide as a signaling molecule in the cardiovascular system.5
The medical significance of INO as a selective pulmonary vasodilator rests on its characteristic of being deliverable as a gas directly to the pulmonary circulation, without systemic adverse effects. Nitric oxide activates guanylate cyclase and converts it into cyclic guanine monophosphate (cGMP). The presence of cGMP at the smooth muscle causes relaxation (Fig. 1).6 When this occurs in the pulmonary vasculature, the result is reduced pulmonary vascular resistance, redistribution of pulmonary blood flow (Q̇p), and a reduction in right-heart work.
Nitric oxide (NO) activates guanylate cyclase, which converts into cyclic guanine monophosphate (cGMP). The presence of cGMP at the smooth muscle causes relaxation. NOS = NO synthase. ACh = acetylcholine. PA = pulmonary artery. Hb = hemoglobin. met Hb = methemoglobin. LA = left atrium. (From Reference 6, with permission.)
The vasodilatory effect of INO redistributes pulmonary blood to lung regions where ventilation is more efficient, thus improving ventilation/perfusion match and oxygenation.7 When INO is delivered to better-ventilated lung regions, blood flow is redistributed to maximize gas exchange. Once nitric oxide enters the circulation, it quickly combines with hemoglobin and forms methemoglobin, preventing systemic effects, and, thus, making it a selective pulmonary vasodilator. This property alone makes INO a very appealing therapeutic agent and a focus of research for many pulmonary disorders.
Indications
The only current FDA-approved indication for INO is for the treatment of term neonates with acute hypoxic respiratory failure associated with pulmonary hypertension, to improve oxygenation and therefore avoid extracorporeal membrane oxygenation (ECMO) and lower mortality. All other uses are considered off-label. It should be noted that many drugs are used beyond their FDA-approved indication as science discovers new applications and indications. The controversy with INO rests with its substantial cost and limited reimbursement, especially for non-approved indications.
Persistent Pulmonary Hypertension of the Newborn.
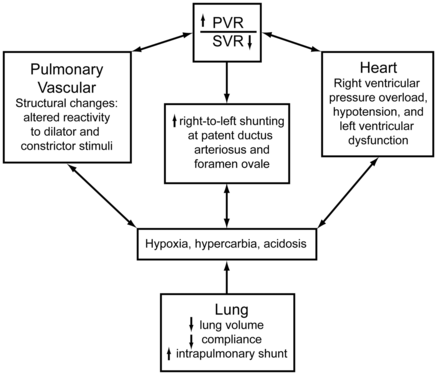
Persistent pulmonary hypertension of the newborn (PPHN) is common in infants with respiratory failure. It is characterized by pulmonary hypertension and extrapulmonary right-to-left shunting across the foramen ovale and ductus arteriosus (Fig. 2).8 In many cases the disease progressively worsens, becoming refractory to standard treatment, including high FIO2, alkalosis, and conventional and high-frequency mechanical ventilation. In severely hypoxemic infants with PPHN, INO rapidly increases PaO2 without causing systemic hypotension. In newborns with hypoxic respiratory failure and PPHN who are candidates for ECMO, multiple clinical trials have shown positive outcomes with INO.9–12 This is an important finding because of the invasive nature, potential for serious complications, and resources required for ECMO, versus INO.
Cardiopulmonary interactions during persistent pulmonary hypertension of the newborn. PVR = pulmonary vascular resistance. SVR = systemic vascular resistance. (Adapted from Reference 8, with permission.)
To assist clinician decision support and policy development, DiBlasi et al recently published evidence-based guidelines to address all aspects of INO therapy in acute hypoxic respiratory failure.13 Using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) scoring system, 22 recommendations were made for the use of INO in newborns (Table 1).
Recommendations From American Association for Respiratory Care Clinical Practice Guideline: Inhaled Nitric Oxide for Neonates With Acute Hypoxic Respiratory Failure
Premature Infants and Bronchopulmonary Dysplasia.
Infants born at less than 34 weeks gestational age often require respiratory support. High mortality in this patient population is due to the effects of prematurity and respiratory distress. Bronchopulmonary dysplasia (BPD) in premature infants is associated with prolonged hospitalization and abnormal pulmonary and neurodevelopmental outcomes. Although improvements in mechanical ventilation strategies and medical therapies, such as high-frequency ventilation and exogenous surfactant, have improved in outcomes in pre-term infants, the incidence of chronic lung disease remains a substantial concerning contributor to morbidity. Nitric oxide is an appealing agent to treat this rather fragile population, because INO improves pulmonary blood circulation and oxygenation, thereby reducing the need for high FIO2 and possibly injurious mechanical ventilation parameters.
Numerous clinical trials have delivered INO to premature infants born ≤ 34 weeks gestational age, for both the treatment and prevention of BPD, along with supporting gas exchange during acute respiratory distress.14–17 But despite various clinical trial designs, dosing strategies, diverse patient populations, and multiple weight classes, there is currently no evidence to support the use of INO in pre-term infants when using death or BPD as an outcome measure. In a systematic review by Donohue et al, 14 randomized controlled trials, which included 3,461 patients, examined mortality, BPD, and short-term and long-term risks of INO.18 Mortality in the neonatal intensive care unit (ICU) did not differ in the infants treated with INO, compared to the control group (risk ratio 0.97, 95% CI 0.82–1.15). BPD at 36 weeks for the INO and control groups also did not differ for survivors (risk ratio 0.93, 95% CI 0.86−1.00). There was no evidence to suggest a difference in the incidence of cerebral palsy (risk ratio 1.36, 95% CI 0.88−2.10), neurodevelopmental impairment (risk ratio 0.91, 95% CI 0.77–1.12), or cognitive impairment (risk ratio 0.72, 95% CI 0.35–1.45). At this time, the administration of INO for premature infants is not supported by the medical literature.
Congenital Heart Disease: Post-Operative Administration.
Although the only approved indication for INO is PPHN in the near-term infant, there is widespread experience and a large body of evidence describing INO's use in the postoperative period after surgical repair or palliation of congenital heart disease.19 A subset of infants and children with congenital heart lesions associated with pulmonary overcirculation are at an elevated risk for pulmonary hypertension in the postoperative period. Although the incidence of pulmonary hypertension in the postoperative period is reported to be 7%, it is associated with a mortality rate of 29%.20
The primary goal of INO in the postoperative period is to reduce elevated pulmonary artery pressure, thereby lowering pulmonary vascular resistance, and improving right-heart function (Fig. 3).19,21,22 However, when subjected to the rigorous methods of the Cochrane Database of Systematic Reviews, there was insufficient evidence to support INO during the postoperative period for outcome measures of mortality, oxygenation, improved hemodynamics, or incidence of pulmonary hypertension.23 However, that recommendation, in large part, is due to the small number of randomized controlled trials that have compared INO to placebo in this patient population. Unfortunately, given the limited data, a definitive recommendation on INO in the postoperative congenital heart disease patient cannot be made.
Change in pulmonary artery pressure, from baseline, in postoperative patients who received inhaled nitric oxide (INO) versus placebo. (Adapted from Reference 19, with permission.)
Acute Respiratory Distress Syndrome.
Important components of acute respiratory distress syndrome (ARDS) are ventilation/perfusion mismatch, intrapulmonary right-to-left-shunt, pulmonary hypertension and accompanying hypoxemia.24–26 The rationale for delivery of INO in ARDS patients is to reduce pulmonary hypertension and direct blood flow toward the better-ventilated alveoli. In 1993, Rossaint et al first described this concept with improved oxygenation and reduced pulmonary artery hypertension in patients with ARDS.27
INO produces vasodilatation in ventilated lung units and decreases pulmonary hypertension, thereby reducing ventilation/perfusion mismatch by redistributing pulmonary perfusion toward ventilated regions and enhancing oxygenation. The importance of delivering INO to well ventilated alveoli cannot be stressed enough. Several reports show a synergistic effect of INO with high-frequency oscillatory ventilation and an elevated mean airway pressure to recruit the lung and provide more surface area for gas exchange.28–30 Simply stated, if the lungs are not open, INO is unable to enter the blood stream and vasodilatation cannot occur.
Multiple clinical trials of pediatric and adult ARDS patients treated with INO have been published.31–39 Generally, 60–80% of ARDS patients respond to INO with a 20% improvement in oxygenation and a 10% reduction in pulmonary artery pressure. Despite these encouraging improvements in oxygenation, when examined in randomized controlled trials in both adult and pediatric patients with ARDS, INO was found to have no effect on mortality or the duration of mechanical ventilation. In a recent systematic Cochrane review, 14 randomized controlled trials, which included 1,303 subjects, showed no statistically significant effect on overall mortality (40.2% vs 38.6%) (risk ratio 1.06, 95% CI 0.93–1.22). Limited data indicated a statistically insignificant effect of INO on duration of mechanical ventilation, ventilator-free days, and ICU and hospital stay.40 Transient improvement in oxygenation, although encouraging, does not reduce mortality and might be harmful. In summary, at this time INO cannot be recommended for patients with ARDS, except as a potential bridge to ECMO, when improved oxygenation and overall clinical stability may be needed for a short period.
Adverse Effects
Abrupt discontinuation of INO can precipitate a rapid increase in intrapulmonary right-to-left shunting and a decrease in PaO2, due to severe rebound pulmonary hypertension. The reasons for this rebound phenomenon are not entirely known, but it may relate to feedback inhibition of nitric oxide synthase activity and/or elevated endothelin-1 level.41,42 In some patients the hypoxemia and pulmonary hypertension may be worse after discontinuing INO than prior to INO.
Several strategies may help avoid rebound during withdrawal of INO. First, to use the lowest effective INO dose (generally ≤ 5 ppm). Second, to not withdraw INO until the patient's gas exchange and hemodynamic status improve sufficiently. Third, to maintain the INO dose at ≤ 1 ppm for 30–60 min before discontinuing. Fourth, to increase the FIO2 by 0.10 when discontinuing INO. Additionally, evidence suggests sildenafil prevents rebound after withdrawal of INO.43 While these are general guidelines, every patient's INO plan must be handled individually. Many institutions have a protocol with specific physiologic end points and a decision pathway to aid clinicians in the safe weaning of INO (Fig. 4).
Representative inhaled nitric oxide (INO) weaning protocol. ABG = arterial blood gas. (Adapted from Reference 19, with permission.)
In the presence of oxygen, INO is rapidly oxidized to nitrogen dioxide. To minimize the production of nitrogen dioxide, both the concentration of oxygen and nitric oxide and the contact time between them should be kept to the minimal amount. The Occupational Safety and Health Administration's safety limit for nitrogen dioxide is 5 ppm for 8 hours.44 Although increased airway reactivity and parenchymal lung injury have been reported in humans with inhalation of ≤ 2 ppm of nitrogen dioxide, toxicity is unlikely at < 40 ppm.45
Increasing the INO concentration may lead to methemoglobinemia. Methemoglobinemia toxicity occurs when INO at higher doses binds with hemoglobin in red blood cells,45 which reduces the blood's oxygen-carrying capacity, which in turn, decreases oxygen delivery and creates a functional anemia. The oxyhemoglobin dissociation curve is shifted to the left, diminishing the release of oxygen from red blood cells to the tissues.
Methemoglobin reductase within erythrocytes converts methemoglobin to hemoglobin. The incidence of methemoglobin is low when the INO concentration is < 40 ppm (and usually much less than 40 ppm). Methemoglobinemia can also be caused by other substances, including nitrates, prilocaine, benzocaine, dapsone, and metoclopramide. Methemoglobin is reported with blood gas CO-oximetry and should be monitored during INO therapy. The normal methemoglobin level is < 2%, and a level below 5% does not require treatment. If the methemoglobin level is increasing, a lower but still effective INO dose may be used. If the methemoglobin level becomes substantial, then INO should be discontinued, and methylene blue administration, which increases reduced nicotinamide adenine dinucleotide-methemoglobin reductase, should be considered. Ascorbic acid can also be used to treat methemoglobinemia.
INO may have adverse hemodynamic effects in patients with preexisting left-ventricular dysfunction and/or mitral stenosis. The acute reduction in right-ventricular afterload may increase pulmonary venous return to the left heart, thereby increasing left-ventricular filling pressure and worsening pulmonary edema.
Delivery Systems
In North America there is only one INO delivery system: INOvent (Ikaria, Clinton, New Jersey). The INOvent connects to the inspiratory limb of the ventilator circuit and provides a constant INO concentration, set by the clinician, over a wide range of minute volume and patient sizes. Monitoring includes the INO concentration, FIO2, and NO2, with accompanying alarms. Although INO is typically delivered in an ICU setting, the device can operate for up to 6 hours on battery, for transport applications.
Alternatives
Although this paper is focused on inhaled medical gases, it would be remiss not to discuss alternatives to INO. A growing number of papers have described other inhaled selective pulmonary vasodilators.46 The compelling basis for these investigations is to safely and effectively deliver pulmonary vasodilators without the economic liability and possible toxicity associated with INO.47
Inhaled prostacyclin analogs such as epoprostenol and iloprost are an alternative to INO.48 Epoprostenol is a nonselective pulmonary vasodilator, so it may contribute to hypotension during intravenous administration. However, when delivered via the inhaled route, Kelly et al found that 50 ng/kg/min improved oxygenation index without systemic adverse effects in neonates who failed INO. Within 1 hour of initiation of epoprostenol, PaO2 increased from 57 ± 6 mm Hg to 100 ± 27 mm Hg (P = .06). Within 2 hours, the mean oxygenation index decreased from 29 ± 5 to 19 ± 7 (P < .05). Of note, no changes were made in the mechanical ventilation pressure settings or inotropic support during the study period.49
Iloprost has been evaluated more than epoprostenol, because iloprost is FDA-approved for inhalation. Iloprost reduced mean pulmonary artery pressure, improved systemic oxygenation, and lowered the ratio of pulmonary vascular resistance to systemic vascular resistance equal to INO.50–52 Recently, Loukanov et al studied aerosolized iloprost versus INO in pediatric patients with left-to-right shunt undergoing intracardiac repair with cardiopulmonary bypass. There was no difference between the groups in the frequency of pulmonary hypertension, mean pulmonary artery pressure, or duration of mechanical ventilation (P < .05).50 These findings are similar to other studies of INO versus iloprost on mean pulmonary artery pressure and postoperative pulmonary hypertension.51,52
Summary and Future Direction
The indications for INO in term infants with PPHN and hypoxemic respiratory failure are well established, and INO may help to avoid the need for ECMO. Multiple clinical trials have demonstrated favorable clinical outcomes. Although INO management guidelines exist, each patient is unique and care should be individualized based on clinical response. Work continues on the optimum dosing and weaning strategies to both optimize and minimize time on INO.
Premature infants do not seem to benefit from INO when considering end points of BPD or mortality. This is in part due to the complexity of variables associated with prematurity that may be beyond the scope of INO.
The use of INO to manage postoperative pulmonary hypertension in patients with congenital heart disease seems to be effective in improving oxygenation and reducing pulmonary artery pressure and pulmonary vascular resistance without systemic adverse effects, which can be seen when vasodilatory agents are administered intravenously. Although not subjected to vigorous testing during large multicenter randomized controlled trials, INO is commonly administered for this purpose.
The role of INO as an adjunct to mechanical ventilation in pediatric patients with ARDS remains controversial. The question remains whether to use INO beyond its approved indication. Although INO has scientific and physiologic merits by increasing PaO2 and reducing pulmonary artery hypertension, no study has demonstrated improved outcomes in patients with ARDS. The PaO2 increase may allow the patient to get through a critical phase of ARDS, but the evidence on INO's effect on survival and other outcomes is generally not sufficient to justify INO in most ARDS patients.
Future INO research will evaluate INO's role in lung injury, inflammation, and other disease states. As more knowledge is gained about the interaction of INO and the human body, new and promising therapies may emerge to treat cardiopulmonary diseases. However, alternatives to INO will have an increasing role as selective pulmonary vasodilators, because of efficacy and cost considerations.
Heliox
Helium and oxygen mixture (heliox) has been used for clinical purposes since 1934.53 Heliox has been studied and reported to be effective in a variety of respiratory conditions such as upper-airway obstruction, status asthmaticus, decompression sickness, post-extubation stridor, bronchiolitis, and ARDS.54–58 Barach first described the positive effects of heliox for treating patients with asthma and airway obstruction. The observation of reduced work of breathing (WOB) immediately after treatment with heliox is the rationale for current clinical applications. From the 1940s until the 1980s, heliox all but faded from medical literature. An increased mortality rate in patients with status asthmaticus in the 1980s returned heliox to the clinical arena.
By itself, helium, an inert gas, is odorless and tasteless, and it does not support combustion or react with biologic membranes. Helium is 86% less dense (0.179 g/L) than room air (1.293 g/L). It is 7 times lighter than nitrogen, and 8 times less dense than oxygen (Table 2). The only gas with a lower density is hydrogen, which is highly flammable. The lower density of helium reduces the Reynolds number associated with flow through the airways. The Reynolds number represents the relationship between the airways radius and the velocity, density, and viscosity of the gas and is expressed as:
Gas Density and Viscosity of Nitrogen, Oxygen, Air, and Helium
Heliox converts areas of extreme turbulence and makes these areas less turbulent. Additionally, heliox converts some areas of turbulence to areas of more efficient laminar flow. Thus, heliox improves the efficiency of gas flow through narrowed orifices.
Heliox decreases WOB in patients with increased airway resistance.59 However, heliox does not treat airway resistance, but, rather, reduces the inspiratory pressure of the patient or ventilator required for a given gas flow. The effort required to move a volume of gas to the alveolus is reduced by up to one third when breathing helium rather than nitrogen. Additionally, helium enhances the effect on carbon dioxide elimination, which diffuses approximately 4 times faster with heliox than with a nitrogen/oxygen mixture. Overall, the unique physical properties of helium promote less turbulent gas flow, decreased airway resistance, and decreased WOB in patients with air-flow obstruction. Because helium is an inert gas, not known to interact with human metabolism, it can be used on any patient without adverse effects.
Clinical Indications
The use of heliox for any clinical condition remains controversial because there have been no large randomized controlled trials to determine its indications and limitations. Most clinical trials of heliox have enrolled fewer than 30 patients. However, based on a recent review of heliox use in pediatric patients, no complications have been reported, assuming it is correctly administered.59
It is extremely important to note that heliox has no therapeutic benefit to treat underlying disease. Instead, heliox is used solely to reduce airway resistance and decrease WOB until other therapies (eg, oxygen, bronchodilators, steroids, antibiotics) are effective. Several studies have found rapid reduction in symptoms with heliox but an equally quick return to baseline when heliox was withdrawn.59–63
The use of heliox to manage upper-airway obstruction is well described. Since the first described benefits of heliox by Barach in 1934, several other studies have highlighted the rapid and dramatic response to heliox in patients with upper-airway obstruction. Fleming and colleagues studied normal subjects breathing through resistors and found significant improvement in pulmonary function tests with heliox.60 Heliox increased the gas flow past an area of airway obstruction.
However, heliox has been well described in other causes of acute respiratory failure. Heliox is recommended as a useful adjunct in patients with severe asthma, both for spontaneous breathing and mechanical ventilation. Gluck et al administered heliox to 7 patients with status asthmaticus intubated for respiratory failure.57 All patients experienced a significant reduction in PaCO2 and peak airway pressure within 20 min, and increased tidal volume (VT). Gluck et al concluded that heliox may help reduce the risk of barotrauma and improve overall ventilation. The theorized physiology of heliox and asthma is that improved alveolar ventilation promotes rapid washout of alveolar carbon dioxide and enhanced removal of arteriolar carbon dioxide.
Heliox also increases the deposition of inhaled particles to the distal airways in patients with severe asthma. Anderson et al found that radiographically labeled particles delivered with heliox are better retained in patients with asthma than in healthy volunteers.64 They believed that the difference in particle deposition was because of decreased turbulence with heliox. They also suggested that the improved deposition would be more pronounced in patients with a greater degree of obstruction. Patients with severe asthma may be better served by delivering aerosolized medications with heliox rather than room air or oxygen-enriched air.
To date, limited data exist for the use of heliox in ARDS. Theoretically, in patients with ARDS, during mechanical ventilation, heliox should allow lower peak inspiratory pressure and plateau pressure, and increase VT, minute volume, and peak expiratory flow. The resulting gas-exchange benefit may reduce PaCO2 and improve oxygenation. Because these trials have yet to be conducted, the benefits of heliox and ARDS are only speculative. Interestingly, heliox has been used during high-frequency ventilation.61,62 When systematically tested, the benefits of improved gas exchange resulted from increased VT during high-frequency ventilation rather than from improved gas-flow efficiency.63
Other Considerations
Patients receiving heliox therapy are usually located in the emergency department, ICU, or a monitored step-down unit. Because of the severity of illness that requires heliox, these patients must be closely monitored. Non-intubated patients must be educated on the importance of continuously wearing the mask during heliox. Usual monitoring includes pulse oximetry, peak expiratory flow, heart rate, respiratory rate, arterial blood gases, peak inspiratory pressure, and VT. Careful attention must be given to the amount of heliox available, because unexpected disruption in gas delivery can have serious consequences. When heliox is discontinued abruptly, the patient may decompensate quickly.
Heliox discontinuation usually involves simply turning the heliox off and watching for signs of respiratory distress, elevated peak inspiratory pressure, or decreased VT. Heliox weaning and discontinuation must be performed after other therapeutic agents have had adequate time to work. If the patient tolerates a brief interruption of heliox without any respiratory compromise, the patient is likely to tolerate heliox discontinuation. A rebound phenomenon is very unlikely.
Delivery Systems
Heliox is commercially available in H, G, and E size medical gas cylinders. Helium and oxygen are typically blended to percentage concentrations of 80:20, 70:30, and 60:40, respectively. Clinicians must never use 100% helium because of the risk of delivering an FIO2 <0.21. Gas regulators manufactured specifically for helium must be used to deliver heliox safely and accurately.
Caution must also be used with heliox and medical devices. Oxygen and air flow meters do not correctly measure heliox flow because of the difference in gas density. The clinician should calculate the predicted heliox flow for accuracy. For example, 80:20 heliox is 1.8 times more diffusible than oxygen. For every 10 L/min gas indicated on a standard flow meter, the actual delivered gas is 18 L/m.
Heliox can negatively affect nebulizer functioning if the appropriate flow is not utilized. An inappropriate heliox flow rate can result in smaller particle size, reduced output, and longer nebulization time.64,65 When heliox is used to power a nebulizer, the flow should be increased by 50–100% to ensure adequate nebulizer output. With an appropriate flow, heliox improves aerosol delivery during mechanical ventilation.
While some mechanical ventilators are calibrated to deliver heliox, others are not. If a ventilator is not calibrated to deliver heliox, the density, viscosity, and thermal conductivity of heliox may interfere with ventilator functioning. Caution must be used with any mechanical ventilator that is not calibrated for heliox, because a malfunction may occur with gases other than air and oxygen,66,67 and the delivered and exhaled VT measurement can be severely altered.68 A mechanical ventilator must be approved, or at least adequately bench-tested, to ensure safe operation with heliox. It is imperative to consult the ventilator's operation manual and contact the ventilator manufacturer to guarantee the safe use of heliox.
Heliox is relatively expensive compared to oxygen, but is certainly much less expensive than some other respiratory therapies such as mechanical ventilation and INO. Heliox's benefits are realized only while the patient is breathing heliox, so heliox treatment must be continuous. If a patient needs heliox for more than 48 hours, reevaluate the underlying disease state and consider other therapies. Gas can be conserved by minimizing unnecessary loss to the environment. The number of tanks available at any institution is often limited by space and ordering practice. The space available to store an adequate number of heliox tanks must be taken into consideration prior to initiating heliox therapy so that the patient can avoid an unexpected interruption.
Summary and Future Direction
Heliox is a safe and rapidly acting gas that reduces airway resistance and WOB and improves gas exchange in a variety of respiratory conditions. The therapeutic benefit lies solely in its low density. Published research on heliox dates back over 70 years and suggests that it is useful in airway obstruction, asthma, and possibly other respiratory circumstances. As logical as the use of heliox seems, based on the physics of gas delivery, it is still criticized for a lack of high-level evidence. Properly powering a multicenter randomized controlled trial will be difficult due to the number of patients required.
The ultimate goal of heliox therapy is to reduce respiratory distress and therefore avoid endotracheal intubation or emergency cricothyrotomy. In patients on mechanical ventilation, heliox may improve gas exchange, allow reduced ventilator settings, and aid in liberation from mechanical ventilation. Delivering heliox can be problematic at times because of limited equipment options and storage space. Further studies are needed to determine the role of heliox in patients with ARDS. Future investigations should examine the effect of heliox on duration of mechanical ventilation, hospital stay, and outcome.
Inhaled Anesthetics
Sedative drugs are often administered in the ICU setting to facilitate mechanical ventilation and invasive procedures, and to alleviate fear and anxiety.69 Inhaled anesthetic was first described in 1846, and halothane was popularized in 1956. Typically, sedative and anesthetic medications are administered via intravenous infusion in the ICU. Conversely, inhalation is the primary route of anesthesia in the operating room. Typically used inhaled anesthetics include halothane, isoflurane, desflurane, and sevoflurane.70 These agents are volatile liquids, specifically designed to provide anesthesia and amnesia during surgery in the operating room.
Clinical Indications
The commonly reported indications for inhaled anesthetic outside the operating room are sedation of mechanically ventilated patients, and treatment of status asthmaticus and status epilepticus when conventional therapies prove ineffective.70,71
The bronchodilator effects of inhaled anesthetic have been suggested. The mechanisms by which this action takes place are many and include: β-adrenergic receptor stimulation, direct relaxation of bronchial smooth muscle, inhibition of the release of bronchoactive mediators, antagonism of the effects of histamine and/or methacholine, and depression of vagally mediated reflexes.72,73 The first report of the treatment of status asthmaticus with inhaled anesthetic was in 1939.74 The descriptions of inhaled anesthetic for status asthmaticus have been anecdotal and retrospective, because the strategy is usually employed when other therapies fail to yield adequate gas exchange.
Shankar et al described a retrospective case series of 10 children (ages 1–16 years) who received isoflurane for life-threatening status asthmaticus. Isoflurane significantly reduced PaCO2 (P = .03) and improved pH (P = .03) within 2 hours. Isoflurane was administered for a median duration of 35 hours. The isoflurane peak concentration range was 0.5–1.5%. Nine patients survived and were discharged from the hospital.75 Wheeler et al described a case series of 6 patients (ages 14 months to 15 years) in status asthmaticus who failed conventional management and received inhaled isoflurane. The group exhibited a significant increase in pH (P = .043), and significant decreases in PaCO2 (P = .03) and peak inspiratory pressure (P = .03). All 6 patients were successfully extubated and discharged from the hospital without apparent sequelae.76
In some children with status epilepticus, seizures persist despite treatment with anticonvulsant medications, and alternative approaches have included inhaled anesthetic. Although inhaled anesthetic's mechanism of action in treating status epilepticus is not well understood, the antiepileptic effects of isoflurane may be attributable to the potentiation of inhibitory pathways.77 Interestingly, isoflurane and desflurane produce dose-dependent electroencephalogram changes, whereas sevoflurane may induce epileptiform discharges at a therapeutic level.
In a retrospective case series of 7 patients with status epilepticus, Mirsattari et al reported that seizures were consistently terminated within several minutes of initiating inhaled anesthetic (6 patients received isoflurane, 1 received desflurane). The isoflurane concentration range was 1.2–5.0%, and it was administered for a mean 11 days. All the patients experienced hypotension that required vasopressor support. Three patients died, but the 4 survivors had good or excellent outcomes.78
The largest case series to date of inhaled anesthetic in patients with status epilepticus included 9 patients, including 6 children.79 Isoflurane was used for 1–55 hours and stopped the seizures in all the patients. However, seizures resumed on 8 of 11 occasions when isoflurane was discontinued. Hemodynamic support, including fluid administration and vasopressors, was required in all the patients due to hypotension. Six of the 9 patients died, and the survivors had substantial cognitive deficits.
Sedation with volatile anesthetics in the critical-care environment are usually handled on a case-by-case basis, because of the lack of any published clinical practice guidelines. Such use of inhaled anesthetic is uncommon. To date, no randomized controlled trials have been published on inhaled anesthetic in neonatal or pediatric patients outside the operating room environment. Several anecdotal case series have been published. Arnold et al described successful sedation with isoflurane in 10 pediatric ICU patients ages 3 weeks to 19 years, who had been difficult to sedate with other agents.71 Isoflurane was delivered for days up to a month. However, half of those patients had adverse events, including impaired renal or hepatic function, and withdrawal syndromes. Other case reports describe the long-term use of isoflurane to facilitate sedation during mechanical ventilation in 4 patients.80,81 The total time on isoflurane was up to 8 days, and one of the 4 patients experienced adverse events. The lack of a large body of evidence represents the rarity of utilizing inhaled anesthetic in the ICU.
Clinicians must consider the risk/benefit ratio of inhaled anesthetic, including the end-organ effects,82 such as decreased mean arterial blood pressure and depressed myocardial contractility. The decrease in mean arterial blood pressure may reduce blood flow to other organs systems, such as the renal and the hepatic. Secondary effects on end-organ function may result from the metabolism of inhaled anesthetic and the release of inorganic fluoride.
Delivery Systems
Inhaled anesthetics present a unique logistical challenge when delivered outside of the operating room, because the necessary equipment, trained personnel, and clinical expertise may not be readily available in the ICU. Inhaled anesthetic is delivered via a vaporizer incorporated into or attached to an anesthesia workstation. Each inhaled agent requires a specific vaporizer, because each agent requires a different pressure to vaporize. Vaporizer technology compatible with typical mechanical ventilators has been described83 and might be useful in an adult ICU, but a minimum VT of 350 mL is required, thus making it generally not applicable with neonatal and pediatric patients.
Additionally, inhaled anesthetic delivery requires effective gas-scavenging to protect clinicians and visitors. If inhaled anesthetic is delivered via a mechanical ventilator, consult the operator's manual for instructions on the use of inhaled anesthetic and proper gas-scavenging. This is an issue of device functionally and environmental safety.
Other Considerations
Inhaled anesthetics are typically delivered by specially trained anesthesia clinicians inside the confines of the operating suite. Most critical care practitioners are not thus trained. The delivery of inhaled anesthetics must be the responsibility of clinicians qualified and trained in the set up, handling, and delivery of inhaled anesthetic and related equipment: typically, anesthesiologists. Extreme caution must be taken when delivering inhaled anesthetics outside of their intended use or with equipment not designed for inhaled anesthetic.
Summary and Future Direction
Despite the case reports of inhaled anesthetic use for sedation in the ICU, the technique has never become a standard of care because of concerns about equipment availability, clinical experience and expertise, and unknown consequences to organ systems. There are no guidelines for the use of inhaled anesthetic in the ICU, and special considerations in this setting include equipment, gas-scavenging, and monitoring, which must be clearly determined before implementation. These limitations make the use of inhaled anesthetic in the ICU a rare occurrence.
Inhaled Carbon Dioxide
While one of the major goals of mechanical ventilation is to provide appropriate carbon dioxide elimination, a small subgroup of patients may benefit from the addition of supplemental CO2 to the inspired gas. Hypercarbic gas mixtures may benefit infants with excessive Q̇p related to certain congenital cardiac lesions, such as hypoplastic left-heart syndrome. To achieve pulmonary vasoconstriction, inhaled carbon dioxide has been used to decrease pulmonary circulation, and thus increase systemic blood circulation, in neonatal patients with single-ventricle physiology (eg, hypoplastic left-heart syndrome) in the preoperative and postoperative periods.84 This technique increases the pulmonary vascular resistance, thereby improving systemic blood flow (Q̇s).
The important physiologic condition in hypoplastic left-heart syndrome is the fragile balance between Q̇p and Q̇s. Alteration in pulmonary vascular resistance and systemic vascular resistance will generally affect Q̇p and Q̇s. The relationship between the blood flow of the 2 circulations is usually expressed as a ratio: a Q̇p/Q̇s of substantially greater than 1.0 results in unfavorable effects, including metabolic acidosis, hypoperfusion, and potentially shock. Conversely, a Q̇p/Q̇s of substantially less than 1.0 leads to decreased Q̇p and potentially systemic hypoxemia. A clear understanding of the anatomy, physiology, and therapeutic interventions used to balance Q̇p and Q̇s is essential in caring for patients with hypoplastic left-heart syndrome, but is beyond the scope of this paper. Patients receiving supplemental CO2 require close monitoring with pulse oximetry, capnography, and cardiorespiratory physiologic monitoring.
Clinical Indications
It should be stressed that the use of hypercarbic therapy for infants with single ventricle physiology has become exceedingly rare because of recent advances in the management of these preoperative and postoperative infants. Preoperatively, most infants with this complex physiology can remain extubated and are able to balance their Q̇p/Q̇s without intervention. In postoperative patients, current surgical and medical management of these fragile infants has advanced such that supplemental CO2 is rarely indicated. Further discussion of this topic is beyond the scope of this review.85,86
Delivery Systems
There are no commercially available delivery systems for supplemental CO2 during mechanical ventilation. The delivery of supplemental CO2 is typically via the inspiratory limb of a conventional mechanical ventilator. The mechanical ventilator acts as a blender. The percentage of CO2 in room air is approximately 0.03% or 0.22 mm Hg. The therapeutic range of delivered CO2 is generally 1–4% or 8–30 mm Hg.87 Successful use of a medical gas blender in conjunction with the mechanical ventilator has been described.87–89 The delivery of CO2 seems simple, but vigilance is required in monitoring the inspired CO2 concentration, the FIO2, and PEEP created by the additional gas flow introduced into the ventilator circuit. As a general rule, patients who are managed with supplemental carbon dioxide require sedation and neuromuscular blockade because CO2 greatly stimulates the respiratory drive in an attempt to return to normocapnia.
Summary and Future Direction
The use CO2 for optimizing Q̇p/Q̇s in patients with hypoplastic left-heart syndrome has been described in several cases and is based on physiologic principles. Historically, the use CO2 has proven clinically beneficial in optimizing intracardiac shunting by increasing pulmonary vascular resistance and promoting Q̇s, but it must be stressed that there are no clinical practice guidelines or high-level evidence from randomized controlled trials. Clinicians must understand the anatomy and physiology related to hypoplastic left-heart syndrome and the technical aspects of supplemental CO2 administration to provide safe and effective care. However, as described above, advances in medical and surgical management of this population has largely rendered this technique obsolete.
Hypoxic Gas Mixtures
Similar to supplemental CO2, hypoxic gas mixtures (ie, subatmospheric oxygen concentration, FIO2 < 0.21) have been used to increase pulmonary vascular resistance and optimize Q̇s for infants with single-ventricle physiology (ie, hypoplastic left-heart syndrome and its variants). Reducing the FIO2 below 0.21 results in alveolar hypoxia, pulmonary vasoconstriction, and increased pulmonary vascular resistance. Clinically, hypoxic gas is created by adding nitrogen while the FIO2 is set at 0.21. This reduces FIO2 to 0.14–0.20.
Tabbutt et al conducted a prospective randomized crossover trial of hypoxic gas (FIO2 0.17) versus supplemental CO2 (CO2 concentration 2.7%) in 10 patients. Arterial and superior-vena-cava CO-oximetry and cerebral oxygen saturation measurements were made at the end of each gas mixture (10 min) and recovery period (15–20 min). Q̇p/Q̇s was decreased with both the hypoxic gas (2.55 ± 0.48 vs 3.36 ± 0.46, P = .056) and supplemental CO2 (2.19 ± 0.55 versus 3.11 ± 0.45, P = .026), compared to baseline.90
Questions remain regarding the cerebral effects and safety of hypoxic gas that results in SpO2 < 80%. Toiyama et al evaluated cerebral regional oxygen saturation, and middle-cerebral-artery blood flow and PRV in 8 patients. The average cerebral regional oxygen saturation was 67.3% before hypoxic gas ventilation, and increased to 69.4%, 69.1%, and 70.7% at 1, 12, and 24 hours after the initiation of the hypoxic gas mixture, respectively. Middle-cerebral-artery blood flow significantly increased in the diastolic phase, and an index of vascular resistance significantly improved, from 0.80 to 0.68, within 12 hour of therapy.91
Only one study has evaluated the impact of combining supplemental CO2 and hypoxic gas. Keidan et al used a gas with an FIO2 of 0.18 and 3% CO2 in 12 patients to examine the synergistic influence on hemodynamics. The combination had an additive effect. Ten of the 12 patients showed a positive response (systolic blood pressure increase of ≥ 10 mm Hg). Interestingly, only 3 subjects showed a positive response in the postoperative period when CO2 was added to the hypoxic gas.84
Clinical Indications
In the context of neonatal and pediatric respiratory care, hypoxic gas is indicated only for the preoperative and postoperative support of patients with hypoplastic left-heart syndrome. The use of hypoxic gas, or supplemental CO2, or neither is dependent on clinical expertise, the patient's condition, the precise physiologic objectives, and surgical preparedness. It is difficult to assess the prevalence of the use of hypoxic gas in clinical practice because of a lack of published reports.
Delivery Systems
As with many specialty supplemental blended gases, there are no commercially available systems for the delivery of hypoxic gas, partly because of the low number of patients who require an FIO2 < 0.21. Gas is typically blended externally and then delivered to the patient via mechanical ventilation or oxygen hood. During mechanical ventilation the air hose is typically connected to a nitrogen cylinder equipped with a 50-psi outlet. Equally essential to delivery of hypoxic gas is the accurate and safe monitoring of FIO2. Oxygen analyzers that measure and alarm for FIO2 as low as 0.15 have been evaluated.92 FIO2 must be continuously monitored and the alarms must be set within a very narrow range. An error in gas delivery could have catastrophic consequences, as rapid or substantial fluctuations in Q̇p/Q̇s influence oxygen delivery and organ perfusion.
Summary and Future Direction
The clinical outcomes of patients with hypoplastic left-heart syndrome may depend on the delicate management of Q̇s and Q̇p. Supplemental oxygen, a potent pulmonary vasodilator, is avoided in the management of hypoplastic left-heart syndrome. Hypoxic gas artificially decreases the FIO2 to < 0.21. Although the number of published reports and patients have been small, hypoxic gas does induce hypoxic vasoconstriction and thus increases pulmonary vascular resistance during the management of hypoplastic left-heart syndrome. Additionally, it is believed that hypoxic gas does not affect the central nervous system, as measured by cerebral blood flow. Future research should focus on long-term effects.
Technically, the delivery of hypoxic gas mixture is straightforward but typically requires an override of some of the safety features of most mechanical ventilators. Although the physiologic justification for hypoxic gas is understood, extreme caution must be exercised when using FIO2 < 0.21. As with any blending of medical gases in respiratory care, adequate training of clinicians, patient safety, and monitoring are paramount. However, as is the situation for supplemental carbon dioxide, advances in medical and surgical management of infants with single-ventricle physiology (ie, hypoplastic left-heart syndrome and its variants) have largely rendered hypoxic gas mixtures obsolete.
Inhaled Carbon Monoxide
The most recent inhaled gas receiving research attention for possible therapeutic application is carbon monoxide (CO).93,94 CO is best known as a toxic element of cigarette smoke and a major component of air pollution throughout the world. Inhaled CO binds with blood hemoglobin to form carboxyhemoglobin, which disrupts the normal oxygen transport. Carbon monoxide is odorless and colorless. Overexposure symptoms include headache, dizziness, heart palpitations, weakness, confusion, nausea, convulsions, unconsciousness, and, ultimately, death. However, scientific investigation has discovered that at a concentration of 250–1,000 ppm, CO is an important vascular paracrine factor. Recently, experimental studies revealed benefits from inhaled CO on the progression of various types of pulmonary disorders, using in vivo models of ventilator-induced lung injury, aspiration, hyperoxia, and ischemia-reperfusion.95–99
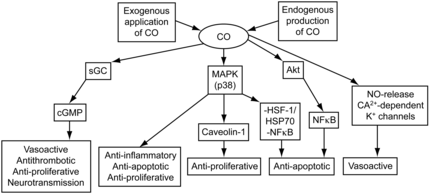
The physiologic basis for benefit from inhaled CO is the recent discovery of the stress protein heme oxygenase-1, which degrades heme to biliverdin-IX alpha, CO, and iron. At a low concentration, CO may provide cyto-protective and tissue-protective effects involving the inhibition of inflammatory, proliferative, and apoptotic signaling (Fig. 5). Lung protection by heme oxygenase-1 was demonstrated in vitro and in vivo in several models of sepsis and acute lung injury. Published studies have also examined the protective effects of pharmacologic or inhaled CO therapy in animal models of acute lung injury and sepsis. CO has shown therapeutic potential in models of oxidative and acid-induced lung injury, ventilator-induced lung injury, endotoxin challenge, and cecal-ligation and puncture-induced sepsis.100
Carbon monoxide (CO) signal transduction pathways. sGC = soluble guanylate cyclase. MAPK = mitogenactivated protein kinase. NO = nitric oxide. cGMP = cyclic guanine monophosphate. HSF = heat shock factor. HSP = heat shock protein. NFκB = nuclear factor-κB. (Adapted from Reference 93, with permission.)
Clinical Indications
Because inhaled CO is only investigational at this point, there are no published guidelines for indications. Any use of inhaled CO should be performed with the approval of an institutional review board that governs human-subjects research.
Delivery Systems
Ikaria developed the Covox DS device (Fig. 6) for investigational delivery of CO, and it is the only device designed specifically for CO administration.101 It delivers CO in proportion to body weight (mg/kg/h), independent of the respiratory rate, and delivery occurs during the inspiratory phase of each breath. CO can be delivered via nasal cannula or injected into the ventilator circuit. The delivery of CO in the first half of inspiration is determined by the dose setting and the respiratory rate (Fig. 6).
A: The Covox DS delivery system for inhaled carbon monoxide. Covox (carbon monoxide for inhalation) and Covox DS are an Investigational Drug and Device, respectively, and are limited by Federal law to investigational use. B: Carbon monoxide concentration with the Covox DS delivery system. (Courtesy of Ikaria.)
Summary and Future Direction
In the past, CO has been considered solely as a toxic substance. However, recent research indicates protective effects from low-concentration exogenous CO for clinical conditions such as organ transplantation, ischemia/reperfusion, inflammation, and sepsis. Human data are lacking, and clinical studies have not been conducted in neonates or children. Randomized controlled clinical trials are needed to clarify whether inhaled CO is safe and effective for various conditions in the ICU. Although CO has had much research attention in preclinical studies, it is not currently available for patients, except in approved clinical research trials.
Hydrogen Sulfide
Hydrogen sulfide (H2S) is a colorless, highly flammable, and water soluble natural gas with the distinctive odor of rotten eggs. Although H2S is usually considered a toxic gas, it was recently discovered to be an important signaling molecule in many physiologic systems, including nervous, inflammatory, and cardiovascular. Joining INO and CO, H2S has been labeled “the third endogenous gaseous transmitter” because of the common characteristic of the ability to bind with hemoglobin.102,103
The theoretical benefit of inhaled H2S gas is to achieve a “suspended animation.” This hibernation-like metabolic state is characterized by a reduction of energy expenditure, which may improve the balance between oxygen delivery and consumption and may help sustain tissue viability in critically ill patients who are in a low-cardiac-output state.104,105 The findings of benefit from H2S are still very preliminary and from small animal models but do raise the possibility of clinical application.
Clinical Indications
Currently there are no clinical indications for the administration of H2S in humans. While the other gases listed in this paper have undergone clinical trials, H2S is still in the pre-clinical phase of investigation. However, inhaled H2S has been studied in various models of shock resulting from hemorrhage, ischemia-reperfusion, endotoxemia, and bacterial sepsis. One report in mice found the inhalation of H2S during mechanical ventilation prevented ventilator-induced lung injury and displayed anti-inflammatory effects by limiting cytokine release and neutrophil transmigration.106 The dosing has been 20−150 ppm H2S over periods of up to 10 hours.
Summary and Future Direction
Over the last few years the role of H2S as a physiologic messenger has been studied in in vitro and in vivo experiments. Although the preclinical data describing H2S-induced “suspended animation” is promising as a new therapeutic adjunct for the management of shock and other critical care conditions, extensive further investigation is required prior to any clinical application. Issues to be evaluated include toxicity and dosing strategies.
Summary
Inhaled medical gases are ingrained in the care of neonatal and pediatric patients. Whether to improve life-threatening derangements in gas exchange or to provide physiologic benefits while other therapeutic interventions are explored, inhaled gases other then oxygen and nitrogen will continue to play an important role in the respiratory care of ICU patients. Clinicians must be aware of the pros, cons, safety, technical aspects of delivery, and potential hazards of each gas.
Discussion
Phelan:*
For hypoplastic kids, can you comment on using CO2 versus 16% O2 to manage pulmonary blood flow?
Gentile:
I'm not aware of any new studies, but several found that CO2 is better. However, both of those combinations always worried me, because if you were blending in CO2, and somebody turned the knob the wrong way at 3:00 am, it could be disastrous. I have the same concern about hypoxic gas mixtures.
Rogers:†
At least the CO2 method allows you to still provide adequate oxygen to the brain. However, CO2 delivery is more problematic, because few CO2 analyzers can be used at the bedside. An end-tidal CO2 monitor won't work. You have to have some kind of continuous CO2 measurement device.
Cheifetz:
I have an important comment about carbon dioxide therapy. Years ago a common clinical debate about single-ventricle patients (ie, with hypoplastic left-heart syndrome) was carbon dioxide therapy versus controlled hypoxia. In the past few years we've moved away from both CO2 and controlled hypoxemia. We've learned that the best option is usually neither CO2 nor controlled hypoxia, because these infants are able to adjust to their physiology and balance their pulmonary systemic blood flow without our help. We learned that infants with single-ventricle physiology are smarter than we are. Today in the preoperative period we do not intubate the majority of these infants, and we allow them to breathe room air without mechanical ventilation.
Willson:
I'm always a little taken aback that people use inhaled anesthetic in the neonatal ICU or for asthma or sedation. I would caution people about long-term exposure to inhaled anesthetic. The operating room has a faster turnover of air than an ICU room or a laboratory.
Gentile:
Yes, you need specialized equipment not typically seen in the ICU. Gas-scavenging equipment is just one component. My advice is to leave the anesthesia in the operating room with the experts who are familiar with the equipment and medications. Another problem with inhaled anesthetic is what to do with the infusions.
Curley:
There was some recent interest, led by Brad Furman, in bringing anesthetics back into the pediatric ICU. Brad developed a system for delivering and scavenging gases, but I haven't heard anything in the pastcouple years. I definitely don't think that this is routine, but some kids would benefit from inhaled anesthetic. It's rapid-acting and useful, especially if they're maxed out on sedation and tolerant and withdrawing, you could put them on anesthesia and withdraw them in a humane way. It can be done, but there are a lot of ramifications regarding nurse, physician, and respiratory therapist staffing and what to do when you have to disconnect the patient and they go from completely anesthetized to wide awake. But an occasional patient could benefit, and we are always quite industrious in doing things our patients need. I don't think we should throw the whole thing away.
Gentile:
What I'm saying is that it's not common and it has to be worked out with the anesthesiology department, and certainly cooperation is required. There can be staffing issues with the busy operating room schedule because of the need for an anesthesiologist or a certified registered nurse anesthetist to be close by the ICU patient.
Curley:
Once the patient is in a steady state, the titrations are minimal. The main thing is who's responsible for filling the vaporizer and managing that.
Gentile:
There have to be systems and policies in place for the few patients per year who require this level of care. There's got to be that system where everyone knows roles, responsibilities, equipment, and medications.
Walsh:
I think one of the main reasons anesthetic gases are being evaluated again is because the anesthesia machines of the past were great gas-passers but poor ventilators, and I think that has changed. The newer-generation anesthesia machines have fairly advanced ventilators that offer pressure support, pressure control, and accurate tidal volume monitoring for even our smallest patients.
Brown:
I've always been perplexed by this. We used inhaled anesthetic 20 years ago, but once we adopted heliox, we never needed inhaled anesthetic. There wasn't ever a patient we couldn't ventilate. I don't think our patients could be that much different from other places, so I fail to see why we'd do something so risky and difficult when we have something so simple and not risky.
Willson:
I've done anesthesia for almost 30 years, it's not just that a scavenging system is required. Trace amounts of anesthesia are constantly released from leaks around the endotracheal tube. Low-dose long-term exposure to inhaled anesthetic increases abortions and malformations. I don't see a rationale for it. I've seen people use inhaled anesthesia for sedation, and if you want to talk about withdrawal, try it after 3 or 4 days of isoflurane “sedation.” In the absence of data—and there are no data—this is not something we should be endorsing.
Gentile:
I agree.
Curley:
When we used it, we put monitoring badges on everyone and measured their exposure, which was definitely within the NIOSH [National Institute for Occupational Safety and Health] standards, so we weren't harming anyone. We established a policy and protocol so we could do it for the occasional patient who needs it.
Branson:
Mike, regarding heliox, my experience is that, if the patient is not intubated, heliox makes a big difference because the patient is doing the work. During mechanical ventilation the ventilator does the work, so it would seem less useful. I take exception to the belief that heliox is safe. I'm preaching to the choir in this room, but I always say that techniques that can only be used by experts should only be used by experts. I think there's a lot of danger in somebody just deciding to add heliox to their Vision circuit to do noninvasive ventilation, or to any ventilator, without verifying operation at the bench. When do you get a dose of heliox that is no longer beneficial? Does it have to be 80:20? Does it have to be 70:30? Can you go even lower on the percentage of helium?
Gentile:
We're worried about how heliox affects the functioning of the equipment. Many manufacturers clearly state, in big bold font in the operator's manual, “Do Not Use Heliox,” because of those problems. But several available ventilators have a heliox delivery feature. The necessary helium concentration has not been fully studied. It's been described, but it's theoretical. Some people say you have to have 70% helium, but there's also evidence that a little whiff will do. When the patient's on the ventilator you're looking for physiologic variables to get better.
We had only tested in the lab how to connect heliox to a Servo 300, but we tried it with one patient, and he got markedly better with heliox, and it didn't interfere with the ventilator at all. That's an “n of 1” study, of course, but his CO2 went from 100 mm Hg down to normal just from adding heliox.
Cheifetz:
Regarding the required helium concentration, we must remember that there is not a “step-off” in the clinical effect of heliox. The benefit of heliox is based in physics, and the gas density has a linear relationship to gas concentration,1–3 so the clinical effect of heliox should be linearly proportional to the percent of helium. In a patient with status asthmaticus who is requiring an FIO2 of 0.8, heliox might still be beneficial. That is, 80% oxygen and 20% helium might have some beneficial gas-flow effects. If so, we can gradually wean the FIO2 and increase the helium concentration.4 We have had success with a very high FIO2 and a low helium concentration. The bottom line is that the effect of helium on gas flow is strictly based on gas laws. It is simply physics.
Myers:
At the 2003 neonatal/pediatric Journal Conference this was my topic.1 Heliox has been around for 80 years and it's still a gas looking for a disease to treat. I think most specialty gases are still looking for diseases to treat. One of my other conclusions was that pulmonary arterial hypertension is still looking for a therapy. I don't think much has changed in 8 years.
Brown:
I concur with Ira: the literature says over and over again that you need a high percentage of helium to be effective, but we've had hundreds of mechanically ventilated patients who we've started on 10% helium and 90% oxygen and it seems to work.
DiBlasi:
I think the major limitation with assessing physiologic outcomes in mechanically ventilated patients during heliox is that we have few objective data, other than blood gases, to determine if it's working. So have we really given heliox a good chance? We finally have ventilators that can deliver it appropriately, and one ventilator, the Avea [CareFusion, Yorba Linda, California] can deliver heliox and measure the WOB with an esophageal balloon catheter. A recent study1 found that heliox significantly improved gas exchange and reduced peak inspiratory pressure and WOB in ventilated neonates with chronic lung disease. That's the first study I've seen in recent years that has reinvigorated my interest in heliox for these patients. I think we need more studies, using the newer technologies.
Brown:
There is quite a bit of work going on, especially in patients with bronchiolitis, on preventing intubation with heliox. There's a ton of work, and the pediatric ICU was our biggest user. I also think there's some promise in the neonatal ICU for preventing intubation with nasal CPAP with heliox. I don't think the story's over on this; it's just finding the right patients.
Fineman:
There is some preclinical data on asthma and INO. I think it was at very high doses, and there are a few dramatic case reports in which children's CO2 went from 130 cm Hg to 40 cm Hg within a few minutes. But nobody seems to be using it. I've tried it a couple times and it had no effect. Is anybody still thinking about it?
Gentile:
Part of it is the cost of INO. During these lean budgetary times, INO is under intense scrutiny. It has an approved indication, but the rest are off-label. We use heliox and other adjunctive therapies, including better nebulization, to try to keep patients off the ventilator.
Walsh:
If NO was charged by the liter, do you think we'd use lower doses, versus per hour?
Gentile:
Yes.
Brown:
Yes.
Walsh:
Doug mentioned the lack of evidence about isoflurane. I had the same thought when I moved to Boston. A large anesthesia group decided to use isoflurane quite often, compared to many centers, in patients with asthma or status epilepticus, who were not responding to the typical therapies. There is also a lack of supporting data in our routine use of terbutaline. Sometimes we're using 2 or 3 times the dose that was proven safe in trying to stop premature contractions in women. That was the argument I made to myself: that maybe isoflurane is not as bad and dangerous as I thought. We know the safety profile pretty well.
Fineman:
Regarding the INO dose, it's clear that if you're using INO to redistribute blood flow and improve ventilation/perfusion matching, you can accomplish that with a very low dose. However, all the animal data and the available human data indicate that for getting the full vasodilator effect it's very dose-dependent. From 5–10 parts per million to 20–40 parts per million it's very dose-dependent. So if you're using it to unload the right heart, as opposed to improving oxygenation, you can make an argument to use a higher dose. I think part of the confusion is that a lot of the times with INO we're looking at saturation or PO2 as an outcome variable, as opposed to what we're doing to the right heart.
Gentile:
Correct. There are 2 distinct uses for INO. One is during ARDS, for hypoxic vasoconstriction. The other is what I call resuscitation by inhalation, where the patient postoperatively starts to hemodynamically deteriorate and INO is used to improve right-heart function. A lower dose can be used, and there's not the emphasis on lung-recruitment, as in ARDS.
Footnotes
- Correspondence: Michael A Gentile RRT FAARC, Division of Pulmonary and Critical Care Medicine, Box 3046, Duke University Medical Center, Durham NC 27710. E-mail: michael.gentile{at}duke.edu.
Mr Gentile presented a version of this paper at the 47th Respiratory Care Journal Conference, “Neonatal and Pediatric Respiratory Care: What Does the Future Hold?” held November 5–7, 2010, in Scottsdale, Arizona.
The author has disclosed no conflicts of interest.
↵* Bill Phelan RRT-NPS, GE Healthcare, Waukesha, Wisconsin.
↵† Mark Rogers RRT, CareFusion, San Diego, California.
- Copyright © 2011 by Daedalus Enterprises Inc.
References
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.
- 11.
- 12.↵
- 13.↵
- 14.↵
- 15.
- 16.
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.
- 26.↵
- 27.↵
- 28.↵
- 29.
- 30.↵
- 31.↵
- 32.
- 33.
- 34.
- 35.
- 36.
- 37.
- 38.
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.
- 56.
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- 64.↵
- 65.↵
- 66.↵
- 67.↵
- 68.↵
- 69.↵
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.↵
- 75.↵
- 76.↵
- 77.↵
- 78.↵
- 79.↵
- 80.↵
- 81.↵
- 82.↵
- 83.↵
- 84.↵
- 85.↵
- 86.↵
- 87.↵
- 88.
- 89.↵
- 90.↵
- 91.↵
- 92.↵
- 93.↵
- 94.↵
- 95.↵
- 96.
- 97.
- 98.
- 99.↵
- 100.↵
- 101.↵
- 102.↵
- 103.↵
- 104.↵
- 105.↵
- 106.↵