Abstract
BACKGROUND: The aim of CPAP and noninvasive ventilation (NIV) is to correct sleep-disordered breathing and nocturnal gas exchange. The aim of the study was to analyze the results of a systematic home pulse oximetry ( ) and transcutaneous carbon dioxide (
) and transcutaneous carbon dioxide ( ) monitoring in stable pediatric subjects on long-term CPAP/NIV or screened for CPAP/NIV weaning, and the consequent interventions in the subjects with abnormal gas exchange.
) monitoring in stable pediatric subjects on long-term CPAP/NIV or screened for CPAP/NIV weaning, and the consequent interventions in the subjects with abnormal gas exchange.
METHODS: The home overnight  and
and  recordings of stable pediatric subjects treated with or weaned from CPAP, NIV, or high-flow nasal cannula between January 2017 and March 2018 were analyzed.
recordings of stable pediatric subjects treated with or weaned from CPAP, NIV, or high-flow nasal cannula between January 2017 and March 2018 were analyzed.
RESULTS: A total of 110 recordings, performed in 79 subjects, median age 6 (interquartile range [IQR] 1.5–14) y, were analyzed. Fifty-two recordings (47%) were performed during NIV, 43 (39%) during CPAP, 2 (2%) during high-flow nasal cannula, and 13 (12%) during a spontaneous ventilation weaning trial from ventilatory support. The quality of recording was excellent in 81% of recordings, 5 recordings (5%) had <4 h of recording time, 5 (5%) had artifacts on the  signal, and 16 (15%) had artifacts on the
signal, and 16 (15%) had artifacts on the  signal. Gas exchange abnormalities were observed in 11 subjects with
signal. Gas exchange abnormalities were observed in 11 subjects with  > 50 mm Hg during ≥ 2% of recording time (n = 8), mean
> 50 mm Hg during ≥ 2% of recording time (n = 8), mean  ≥ 50 mm Hg (n = 6), mean
≥ 50 mm Hg (n = 6), mean  < 35 mm Hg (n = 3), and
< 35 mm Hg (n = 3), and 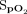 < 90% during ≥ 2% of recording time (n = 2). Consequent interventions were (multiple interventions possible): change of device settings (n = 6), change of interface (n = 2), switched to high-flow nasal cannula (n = 1), and a control recording (n = 2).
< 90% during ≥ 2% of recording time (n = 2). Consequent interventions were (multiple interventions possible): change of device settings (n = 6), change of interface (n = 2), switched to high-flow nasal cannula (n = 1), and a control recording (n = 2).
CONCLUSIONS: A significant number (∼12%) of systematic home  and
and  recordings in stable pediatric subjects treated with CPAP/NIV were abnormal and may be corrected by adequate therapeutic interventions.
recordings in stable pediatric subjects treated with CPAP/NIV were abnormal and may be corrected by adequate therapeutic interventions.
- noninvasive ventilation
- continuous positive airway pressure
- home
- child
- gas exchange
- pulse oximetry
- transcutaneous carbon dioxide monitoring
Introduction
The use of CPAP and noninvasive ventilation (NIV) is increasing in children for the treatment of severe sleep-disordered breathing or nocturnal hypoventilation.1 CPAP consists of the delivery of a constant positive airway pressure that aims to maintain airway patency throughout the entire breathing cycle. CPAP is the accepted standard treatment for severe obstructive sleep apnea that persists despite upper-airway surgery or in a case of impossibility of surgery, mainly in children with underlying conditions such as morbid obesity or malformations of the upper airways.2 CPAP is associated with a reduction in daytime sleepiness and an improvement of neurocognitive performance, behavior disorders, and quality of life.3 NIV consists of the delivery of a positive airway pressure during inspiration with a lower pressure during expiration, with the aim to assist the breathing of the patient. NIV is indicated for disorders that cause disequilibrium in the respiratory balance with hypoventilation, such as neuromuscular diseases, advanced lung disease, or disorders of central drive. In these disorders, NIV unloads or assists the respiratory muscles, or “replaces” central drive. NIV corrects nocturnal hypoventilation and improves sleep architecture, daytime symptoms of nocturnal hypoventilation, and quality of life.4,5
CPAP and NIV may be initiated in the hospital, in an in- or out-patient setting, or at home.6 The aim of the initiation is to explain and acclimatize the patient to his or her treatment and to choose the most appropriate equipment and device settings to correct sleep-disordered breathing. Adequate device settings are checked, ideally by a titration polysomnography or polygraphy, with nocturnal gas exchange comprising a carbon dioxide (CO2) recording once the patient is acclimatized to his or her treatment.7 During follow-up, a polysomnography and/or polygraphy with CPAP or NIV should be repeated at least annually.7 In clinical practice in children, the adequate correction of sleep-disordered breathing should be checked more frequently because of growth, intercurrent events (such as an increase in the adenoid or tonsillar volume or weight gain), and the possibility of a physiologic improvement (such as observed in Pierre Robin syndrome) or deterioration (such as observed in children with progressive neuromuscular diseases).
Because of a limited access to polysomnography and/or polygraphy and a shortage of hospital beds, we developed a routine regular (every 3–6 months) home recording of  and transcutaneous CO2 (
and transcutaneous CO2 ( ) with CPAP/NIV for all patients on long-term CPAP/NIV followed up at our center. Indeed, we have shown that a high number of stable subjects treated with long-term CPAP/NIV remain hypercapnic during sleep despite the lack of clinical symptoms, normal overnight
) with CPAP/NIV for all patients on long-term CPAP/NIV followed up at our center. Indeed, we have shown that a high number of stable subjects treated with long-term CPAP/NIV remain hypercapnic during sleep despite the lack of clinical symptoms, normal overnight  , and normal daytime blood gases, underlying the need for systematic overnight gas exchange recordings.8 We also showed that this monitoring is feasible at home with a trained staff.9 Because a significant number of pediatric patients may be weaned from CPAP/NIV, we also use home
, and normal daytime blood gases, underlying the need for systematic overnight gas exchange recordings.8 We also showed that this monitoring is feasible at home with a trained staff.9 Because a significant number of pediatric patients may be weaned from CPAP/NIV, we also use home  and
and  recordings as a screening for CPAP/NIV weaning before performing an in-hospital polysomnography and/or polygraphy.10,11 The aim of our study was first, to analyze the results of a systematic home
recordings as a screening for CPAP/NIV weaning before performing an in-hospital polysomnography and/or polygraphy.10,11 The aim of our study was first, to analyze the results of a systematic home  and
and  monitoring in a cohort of stable pediatric subjects on long-term CPAP/NIV or screened for CPAP/NIV weaning, and second, to describe the consequent interventions in the subjects with abnormal gas exchange.
monitoring in a cohort of stable pediatric subjects on long-term CPAP/NIV or screened for CPAP/NIV weaning, and second, to describe the consequent interventions in the subjects with abnormal gas exchange.
QUICK LOOK
Current knowledge
The use of CPAP and noninvasive ventilation (NIV) is increasing in children for the treatment of severe sleep-disordered breathing or nocturnal hypoventilation. A close follow-up of children treated with CPAP and/or NIV is recommended to check the efficacy of treatment. Routine monitoring comprises recording of pulse oximetry (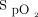 ) and transcutaneous carbon dioxide (PtcCO2) during overnight hospitalizations.
) and transcutaneous carbon dioxide (PtcCO2) during overnight hospitalizations.
What this paper contributes to our knowledge
Home monitoring of combined  and PtcCO2 was feasible and useful for the follow-up of children on long-term CPAP and/or NIV at home and was associated with a decreased hospital burden for subjects and families.
and PtcCO2 was feasible and useful for the follow-up of children on long-term CPAP and/or NIV at home and was associated with a decreased hospital burden for subjects and families.
Methods
Subjects
The study was conducted at the Noninvasive Ventilation and Sleep Unit, Necker University Hospital, Paris, between January 2017 and March 2018. All stable pediatric patients (<18 y old) who were treated with CPAP/NIV or high-flow nasal cannula (HFNC) for at least 1 month or who were weaned from CPAP/NIV or HFNC during the study period were enrolled. Only subjects in whom a complete correction of sleep-disordered breathing was obtained after CPAP/NIV initiation were enrolled. HFNC was initiated as a “rescue” treatment in 2 subjects with severe obstructive sleep apnea who did not adhere to standard CPAP.12 The  and
and  recordings were performed at home as part of the routine follow-up every 1 to 6 months. Indeed, in France, home
recordings were performed at home as part of the routine follow-up every 1 to 6 months. Indeed, in France, home  and
and  recordings are reimbursed to trained staff of home-care providers of children treated with long-term CPAP.13 Demographic and anthropometric data were collected. The study was conducted in agreement with the French regulations and received appropriate legal and ethical approval from the ethical committee CPP Ile de France II, protocol 2013-A00374-41.
recordings are reimbursed to trained staff of home-care providers of children treated with long-term CPAP.13 Demographic and anthropometric data were collected. The study was conducted in agreement with the French regulations and received appropriate legal and ethical approval from the ethical committee CPP Ile de France II, protocol 2013-A00374-41.
 and
and  Monitoring
Monitoring
The routine overnight  and
and  recording with the SenTec digital transcutaneous monitor system (SenTec, Therwil, Switzerland) was performed by trained technicians of the home-care provider in charge of the subject. The technician visited the family in the evening and either explained to the parents how to install the
recording with the SenTec digital transcutaneous monitor system (SenTec, Therwil, Switzerland) was performed by trained technicians of the home-care provider in charge of the subject. The technician visited the family in the evening and either explained to the parents how to install the  and
and  sensor or installed it him- or herself, at the usual bedtime when the subject was falling asleep with his or her CPAP/NIV, and how to take it off after 8 hours to have the drift correction of the
sensor or installed it him- or herself, at the usual bedtime when the subject was falling asleep with his or her CPAP/NIV, and how to take it off after 8 hours to have the drift correction of the  signal. The parents were instructed to note on a recording sheet every problem that occurred during the night. The technician came back on the following morning to download the data and transmitted these by secured e-mail to the hospital team. The results were analyzed within 24 h by the hospital team and transmitted to the family and the home-care provider. Different interventions, depending on the
signal. The parents were instructed to note on a recording sheet every problem that occurred during the night. The technician came back on the following morning to download the data and transmitted these by secured e-mail to the hospital team. The results were analyzed within 24 h by the hospital team and transmitted to the family and the home-care provider. Different interventions, depending on the  and
and  results and the subject’s status, could be decided by the hospital team. These interventions were then performed within 24 h by the technician or the home-care provider, with a consequent control
results and the subject’s status, could be decided by the hospital team. These interventions were then performed within 24 h by the technician or the home-care provider, with a consequent control  and
and  monitoring. This control was preferentially performed at home or in the hospital when a polygraphy with CPAP/NIV was decided or in the case of an imminent scheduled hospitalization.
monitoring. This control was preferentially performed at home or in the hospital when a polygraphy with CPAP/NIV was decided or in the case of an imminent scheduled hospitalization.
Data Analysis
Both  and
and  tracings were visually examined. The duration (in hours) of correct
tracings were visually examined. The duration (in hours) of correct  and
and  signals was recorded. A recording was retained for analysis and considered as good quality when at least 4 h with correct
signals was recorded. A recording was retained for analysis and considered as good quality when at least 4 h with correct  and
and  signals were available. The following data were analyzed: mean
signals were available. The following data were analyzed: mean  , minimum
, minimum  , time spent with an
, time spent with an  < 90%, mean
< 90%, mean  , maximum
, maximum  , time spent with a
, time spent with a  > 50 mm Hg. Nocturnal hypoxemia was defined by a mean
> 50 mm Hg. Nocturnal hypoxemia was defined by a mean  < 92% or ≥2% of the recording time spent with a
< 92% or ≥2% of the recording time spent with a  < 90%. Nocturnal hypercapnia was defined as a mean nocturnal
< 90%. Nocturnal hypercapnia was defined as a mean nocturnal  ≥ 50 mm Hg or ≥2% of the recording time spent with a
≥ 50 mm Hg or ≥2% of the recording time spent with a  > 50 mm Hg. Nocturnal hypocapnia was defined as a mean
> 50 mm Hg. Nocturnal hypocapnia was defined as a mean  < 35 mm Hg.
< 35 mm Hg.
Statistical Analysis
Data are presented as mean ± SD or as median and IQR. Statistical calculations were performed by using the MATLAB R2014b software (Mathworks, Natick, Massachusetts). We used a chi-square statistic test to compare qualitative data. Quantitative data were normally distributed, comparisons were made by using one-way or one factor repeated-measures analysis of variance.
Results
Subjects
During the study period, a total of 110 overnight  and
and  recordings were performed at home in 79 subjects (Table 1). Seventy-one subjects (90%) were treated with CPAP, NIV or HFNC, whereas 8 subjects (10%) had a recording during spontaneous breathing for a weaning trial. The median age of the subjects was 6 (IQR, 1.5-14) y the majority of the subjects had an underlying genetic and/or malformative disorder.
recordings were performed at home in 79 subjects (Table 1). Seventy-one subjects (90%) were treated with CPAP, NIV or HFNC, whereas 8 subjects (10%) had a recording during spontaneous breathing for a weaning trial. The median age of the subjects was 6 (IQR, 1.5-14) y the majority of the subjects had an underlying genetic and/or malformative disorder.
Characteristics of the Subjects
 and
and  Recordings
Recordings
The quality of recording was excellent in 89 of the recordings (81%) (Table 2). Poor quality of recordings was due to a recording time of <4 h in 5% of the recordings, artifacts on the  signal in 5% of the recordings, and artifacts on the
signal in 5% of the recordings, and artifacts on the  signal in 15% of the recordings. No adverse effects, such as skin burns, were observed during the recordings. Thirteen recordings (12%) performed in 11 subjects showed abnormal
signal in 15% of the recordings. No adverse effects, such as skin burns, were observed during the recordings. Thirteen recordings (12%) performed in 11 subjects showed abnormal  or
or  results (Tables 2 and 3; Fig. 1). The median age of these subjects was 12 (1-16) y and all had an associated disorder. The median duration of CPAP/NIV treatment before the
results (Tables 2 and 3; Fig. 1). The median age of these subjects was 12 (1-16) y and all had an associated disorder. The median duration of CPAP/NIV treatment before the  and
and  monitoring was 7 (1-31) months. Six subjects had an apnea-hypopnea index > 10 events/h before CPAP/NIV treatment, and 7 had nocturnal hypercapnia criteria (5 had a mean
monitoring was 7 (1-31) months. Six subjects had an apnea-hypopnea index > 10 events/h before CPAP/NIV treatment, and 7 had nocturnal hypercapnia criteria (5 had a mean  ≥ 50 mm Hg and 2 spent ≥ 2% of the recording time with a
≥ 50 mm Hg and 2 spent ≥ 2% of the recording time with a  > 50 mm Hg). The compliance of the 11 subjects with CPAP/NIV was excellent, with a median compliance of 8 (6.5-9) h per night, with only one subject sleeping <6 h per night with his CPAP device.
> 50 mm Hg). The compliance of the 11 subjects with CPAP/NIV was excellent, with a median compliance of 8 (6.5-9) h per night, with only one subject sleeping <6 h per night with his CPAP device.
Results of the overnight gas exchange recordings performed during various levels of of support. HFNC = high-flow nasal cannula, NIV = noninvasive ventilation.
Quality and Results of the Overnight Gas Exchange Recordings (no. = 110)
Characteristics of the 11 Subjects With an Abnormal Overnight Gas Exchange and Consecutive Interventions
A mean  ≥ 50 mm Hg was observed on 6 recordings, whereas a mean
≥ 50 mm Hg was observed on 6 recordings, whereas a mean  < 35 mm Hg was observed on 3 recordings. Eight subjects spent ≥ 2% of the recording time with a
< 35 mm Hg was observed on 3 recordings. Eight subjects spent ≥ 2% of the recording time with a  > 50 mm Hg and 2 with ≥ 2% of recording time with a
> 50 mm Hg and 2 with ≥ 2% of recording time with a  < 90%. Eight of these 11 subjects were treated with NIV (25% of all the subjects treated with NIV) and 3 were treated with CPAP (8% of all the subjects treated with CPAP). None of the subjects treated with HFNC or on spontaneous breathing had an abnormal recording. The incidence of an abnormal recording was not different between CPAP or NIV users (P = .07).
< 90%. Eight of these 11 subjects were treated with NIV (25% of all the subjects treated with NIV) and 3 were treated with CPAP (8% of all the subjects treated with CPAP). None of the subjects treated with HFNC or on spontaneous breathing had an abnormal recording. The incidence of an abnormal recording was not different between CPAP or NIV users (P = .07).
The following therapeutic interventions were performed after the home recording: an increase in inspiratory pressure (on 6 occasions), change of the interface (on 2 occasions), control recording (4 occasions), and switch to HFNC (1 occasion). These interventions were checked by a control recording at home (6 occasions) or in the hospital (5 occasions). Two subjects with severe nocturnal hypoventilation due to cyphoscoliosis had an in-hospital polygraphy with NIV for a NIV titration study. In 3 subjects, the control was performed in the hospital because of an imminent scheduled hospitalization. After intervention, gas exchanges were normal in 6 subjects, 2 subjects had persistent hypercapnia (one 18-y-old boy with mucopolysaccharidosis and one 9-y-old boy with Down syndrome treated with CPAP in whom CPAP settings could not be improved and with no other therapeutic possibility), one subject had persistent hypoxemia, with 2 subjects were lost to follow-up.
Discussion
This study showed that ∼12% of routine home monitoring of combined  and
and  in a group of stable pediatric subjects on long-term CPAP/NIV, after an adequate initial initiation of CPAP/NIV, were abnormal. Our results highlighted the usefulness of
in a group of stable pediatric subjects on long-term CPAP/NIV, after an adequate initial initiation of CPAP/NIV, were abnormal. Our results highlighted the usefulness of  monitoring because 11 of the 13 abnormal recordings showed abnormal
monitoring because 11 of the 13 abnormal recordings showed abnormal  values without nocturnal hypoxemia. Of note, the normality of the
values without nocturnal hypoxemia. Of note, the normality of the  and
and  monitoring in the 13 subjects during a CPAP/NIV weaning was interesting and may be integrated in the weaning process of CPAP/NIV in children.10
monitoring in the 13 subjects during a CPAP/NIV weaning was interesting and may be integrated in the weaning process of CPAP/NIV in children.10
This study confirmed the feasibility of home recording of 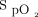 and
and  by trained staff in pediatric subjects on long-term CPAP/NIV. Indeed, the quality of the large majority of the recordings was excellent, as observed in a previous study by our group.9 This recording at home was not only preferred by the parents and subjects but also allowed cost saving because it avoided a hospitalization. Moreover, because the subject remains in his or her own environment, without the stress and disturbance of a hospitalization, the results of the
by trained staff in pediatric subjects on long-term CPAP/NIV. Indeed, the quality of the large majority of the recordings was excellent, as observed in a previous study by our group.9 This recording at home was not only preferred by the parents and subjects but also allowed cost saving because it avoided a hospitalization. Moreover, because the subject remains in his or her own environment, without the stress and disturbance of a hospitalization, the results of the  and
and  recording may even be better.9,14 However, artifacts were more common on the
recording may even be better.9,14 However, artifacts were more common on the  (15%) than on the
(15%) than on the  signal (5%), which is a limitation because the
signal (5%), which is a limitation because the  results were more frequently abnormal.
results were more frequently abnormal.
Interestingly, only 13 recordings (12%) showed abnormal  or
or  values, which is lower than observed in a previous study by our group. Indeed, on a former consecutive cohort, of 65 children treated with CPAP or NIV, 42% of the subjects had nocturnal hypercapnia.8 This difference may be explained by an increase in CPAP and NIV expertise with the use of higher CPAP pressures in young children.15 The information given by the built-in ventilator software, such as objective compliance, leaks, pressure, and tidal volume, also allowed an easier, cheaper, closer, and, consequently, a better follow-up of the subjects.16,17
values, which is lower than observed in a previous study by our group. Indeed, on a former consecutive cohort, of 65 children treated with CPAP or NIV, 42% of the subjects had nocturnal hypercapnia.8 This difference may be explained by an increase in CPAP and NIV expertise with the use of higher CPAP pressures in young children.15 The information given by the built-in ventilator software, such as objective compliance, leaks, pressure, and tidal volume, also allowed an easier, cheaper, closer, and, consequently, a better follow-up of the subjects.16,17
Abnormal  values were more common than abnormal
values were more common than abnormal  values, which underlined the need of systematic
values, which underlined the need of systematic  recording with
recording with  in pediatric patients treated with long-term CPAP or NIV. Of note, abnormal
in pediatric patients treated with long-term CPAP or NIV. Of note, abnormal  values were preferentially observed in the subjects treated with NIV, which is understandable because the indication for NIV is nocturnal hypoventilation. Persistent hypercapnia in these patients warrants an adaptation of NIV settings and, more rarely, a change of device. These findings led to a recent change of the reimbursement legislation in France in 2017 for the monitoring of CPAP in children.13 Nocturnal recordings of
values were preferentially observed in the subjects treated with NIV, which is understandable because the indication for NIV is nocturnal hypoventilation. Persistent hypercapnia in these patients warrants an adaptation of NIV settings and, more rarely, a change of device. These findings led to a recent change of the reimbursement legislation in France in 2017 for the monitoring of CPAP in children.13 Nocturnal recordings of  and
and  are now refunded, at 160 (183 US dollar) for a maximum of 2 recordings per year per subject, for children < 16 y old who are treated at home with long-term CPAP. The usefulness of
are now refunded, at 160 (183 US dollar) for a maximum of 2 recordings per year per subject, for children < 16 y old who are treated at home with long-term CPAP. The usefulness of  and
and  recording is shown by the efficacy of the therapeutic interventions that were performed in all the subjects with abnormal
recording is shown by the efficacy of the therapeutic interventions that were performed in all the subjects with abnormal  and
and  values (Table 3),8 with only 3 subjects having the persistence of nocturnal hypercapnia or hypoxemia.
values (Table 3),8 with only 3 subjects having the persistence of nocturnal hypercapnia or hypoxemia.
Most of the studies on the follow-up of subjects on long-term CPAP/NIV have focused on sleep studies and not overnight gas exchange. In adult subjects, polysomnography with CPAP, performed 3 months after a baseline titration study, showed that ∼25% of the subjects had residual obstructive sleep apnea on CPAP.18 Similar results were observed in another large study, of 905 adult subjects in whom a change in pressure (mostly an increase in airway pressure) was necessary in 58% of the subjects.19 A follow-up polysomnography performed on 42 pediatric subjects treated with long-term CPAP or bi-level ventilation showed that changes in respiratory support settings were recommended in 65% of the CPAP studies and 36% of the bi-level studies.20 However, the results of the polysomnographies and the criteria for changes in the settings, and, in particular, the results of the nocturnal gas exchange were not available and the recommended changes were only fully implemented by the treating physician in patients in 55% of cases.20 A change in respiratory support settings was made in 66% of sleep studies performed in 45 pediatric patients treated with home mechanical ventilation.21 However, again, the detailed results of the sleep studies and the nocturnal gas exchange were not available.
There are no validated guidelines for the monitoring of long-term follow-up of pediatric patients on CPAP or NIV. The timing of the follow-up visits depends on the age and the medical condition of the patient. The feasibility of an overnight gas exchange recording at home coupled with the analysis of the built-in software of the device represents an easy and inexpensive alternative to in-hospital sleep studies. Indeed, we have shown that the analysis of the built-in software that integrates  may be helpful to score residual respiratory events in pediatric patients treated with CPAP.17 However, this “simplified” analysis has not been validated for NIV and is not possible in patients whose weight is below the minimum weight recommended by the manufacturer. Our practice consists in a
may be helpful to score residual respiratory events in pediatric patients treated with CPAP.17 However, this “simplified” analysis has not been validated for NIV and is not possible in patients whose weight is below the minimum weight recommended by the manufacturer. Our practice consists in a  and
and  recording coupled with the analysis of the built-in software of the device 1 month after the initiation, and then every 2 to 6 months, with at least 2 checkups per year, including one full sleep study with CPAP or NIV, but practice at other institutions and in other countries may vary, with no evidence for a superiority of one practice over another.1,22
recording coupled with the analysis of the built-in software of the device 1 month after the initiation, and then every 2 to 6 months, with at least 2 checkups per year, including one full sleep study with CPAP or NIV, but practice at other institutions and in other countries may vary, with no evidence for a superiority of one practice over another.1,22
Our study had several limitations. This was a single-center observational study on a heterogenous population but which was representative of pediatric patients treated with long-term CPAP or NIV at home. Our results reflected the local experience of a specialized pediatric CPAP/NIV team in a tertiary university hospital in a country that benefits from an excellent national home-care network.
Conclusions
A significant number (∼12%) of systematic home  and
and  recordings in stable pediatric subjects treated with CPAP/NIV were abnormal and may be corrected by adequate therapeutic interventions, which underlines the usefulness of routine
recordings in stable pediatric subjects treated with CPAP/NIV were abnormal and may be corrected by adequate therapeutic interventions, which underlines the usefulness of routine  and
and  monitoring.
monitoring.
Footnotes
- Correspondence: Brigitte Fauroux MD, Pediatric Noninvasive Ventilation and Sleep Unit, AP-HP, Hopital Necker, 149 rue de Sèvres, Paris, 75015 France. E-mail: brigitte.fauroux{at}aphp.fr
The authors have disclosed no conflicts of interest.
- Copyright © 2020 by Daedalus Enterprises












