Abstract
BACKGROUND: Pulmonary rehabilitation is an effective treatment for patients with COPD, but patient uptake and adherence to the current offering of center-based pulmonary rehabilitation is modest due to transportation, access, poverty, and frailty, and even more so in the context of the COVID pandemic. Home-based options have been proposed and were found noninferior to center-based rehabilitation; however, there is a lack of home-based programs, and more understanding is needed. We aimed to test the feasibility, uptake, and adherence to a home-based program for COPD rehabilitation with health coaching.
METHODS: We conducted a randomized trial with a wait-list controlled design to evaluate the effects of a home-based program with health coaching on breathlessness in subjects with moderate to severe COPD unable to attend the regular pulmonary rehabilitation program. The 8-week intervention consisted of video-guided exercises to be done 6 times a week and captured with a computer tablet. Health coaching was done weekly over the telephone to review subject activity and symptoms and to provide an opportunity for the subject to define their weekly goals. The primary outcomes were uptake, adherence, and Chronic Respiratory Questionnaire (CRQ) Dyspnea Domain. Secondary outcomes were self-management abilities and CRQ Emotions-Mastery-Fatigue.
RESULTS: 154 subjects with moderate to severe COPD were randomized. Subject adherence was 86% to the proposed 6-times a week exercise routine. There (P = .062) was no significant difference in breathlessness (CRQ dyspnea). There was a significant improvement in self-management abilities (P < .001). The results of the qualitative interviews showed high levels of acceptability of the program.
CONCLUSIONS: The tested home-based rehabilitation program with health coaching was feasible, highly acceptable, showed a high degree of adherence, and improved self-management abilities. This study offers seminal information for home-based rehabilitation programs to design alternative options of rehabilitation to individuals with COPD that cannot attend to the well-established center-based pulmonary rehabilitation. (ClinicalTrials.gov registration NCT02557178.)
Introduction
Pulmonary rehabilitation (PR) is an effective treatment for patients with COPD,1 but patient uptake and adherence to the current offering of center-based PR is modest.2-4 Previous work has demonstrated that 50% of eligible COPD patients do not attend PR, and of those who do begin PR, 50% will not complete the program.5 PR completion is also very low after a hospitalization when PR can have a big impact: only 1.9% of patients received PR within 6 months of their hospital discharge.6,7 PR is a good example of a highly effective intervention that has only modest implementation. The main factors for poor uptake and adherence to PR include transportation, distance, depression, poverty, and motivation to change (current smoking as a behavior is at the top of the list of independent factors associated with low uptake and completion of PR).8 Alternative forms of rehabilitation are needed beyond the well-established and effective center-based PR to increase reach, to adjust to personal needs, and to increase inclusivity.
Several randomized controlled trials suggest the noninferior effectiveness of home-based rehabilitation.9,10 Holland et al10 proposed an intervention that included a home visit by a physiotherapist and weekly telephone calls including personalized support and guidance using Motivational Interviewing. Horton et al9 included 2 telephone calls in a 7-week program and a detailed manual for the participant to follow during the program. Both included training of the providers in Motivational Interviewing like in the intervention presented in this paper.
There is still a need for further understanding of home-based programs to create alternative rehabilitation options that are feasible, can reach most patients, and are safe, acceptable, effective, and potentially billable, all essential ingredients for sustainable implementation. Rehabilitation at its core means restoring function, a goal that needs to be personalized and can be achieved at different intensities of exercise prescription and locations (home or center).
Health coaching also represents an opportunity to improve perceived function and health outcomes. Health coaching is about engaging with people where they are in their journey of coping with a chronic condition, and collaboratively focusing on goals that represent patients’ choices. We previously published the feasibility and effectiveness of telephonic health coaching in decreasing re-hospitalization and sustainably improving the quality of life in subjects with very severe COPD.11,12 Health coaching may therefore represent an effective addition to home-based rehabilitation programs that may add a behavior change component to home-based interventions.
Self‐management interventions like health coaching can improve quality of life and reduce exacerbations in individuals with COPD.13 Such interventions focus on improving these individuals’ confidence and skills in managing symptoms and treatment. Intervention content typically includes action planning and personalized support from a health care professional14,15 or nonlicensed coaches.11 The aim is to utilize health coaching to facilitate behavior change.
Remote monitoring may represent another tool to reach individuals and deliver rehabilitation in the convenience of their own home. Previous research has shown that just monitoring physiologic parameters alone without a meaningful discussion of the findings and planning based on results is ineffective.16,17
We aimed to assess feasibility, uptake, adherence, and patient experience alongside the effectiveness of a home-based rehabilitation program with health coaching. We previously reported the development and pilot testing of this home-based program for COPD rehabilitation that involved both commercially available monitors and telephonic health coaching.2
Quick Look
Current Knowledge
Pulmonary rehabilitation (PR) is an effective treatment for patients with COPD, but patient uptake and adherence to the current offering of center-based PR is modest. Alternative forms of rehabilitation are needed to increase reach and inclusivity; home-based programs and health coaching are potential alternatives to bring PR to more individuals with COPD. There is a need for further understanding of home-based PR programs to create options that are feasible, effective, acceptable to patients, and potentially billable, all essential ingredients for sustainable implementation.
What This Paper Contributes to Our Knowledge
We found that home-based PR with health coaching was feasible and highly acceptable to subjects with COPD, reflected in the high degree of adherence. Home-based PR with health coaching increased self-management abilities and created conditions for a behavior change to a healthier lifestyle. Our results inform our current understanding of home-based PR programs in COPD, and offer the patient's perspectives and the mechanistic effect of the combination of home-based rehabilitation with health coaching on self-management abilities.
Methods
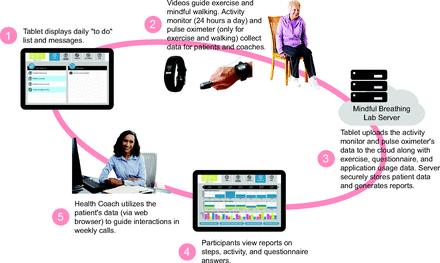
We conducted a randomized trial with a wait-list controlled design to evaluate the effects of a home-based program with health coaching on breathlessness in subjects with moderate to severe COPD unable to attend the regular PR program. The 8-week intervention consisted of video-guided exercises (see the supplementary materials at http://www.rcjournal.com) to be done 6 d per week using an oximeter for detecting exercise-related oxygen desaturation, the measurement of daily steps through an activity monitor and daily self-report of symptoms in a computer tablet provided by this trial. The recommended program did not fulfill the intensity recommended by the American College of Sports Medicine (ACSM) due to safety precautions in the context of the home environment and unsupervised modality. However, this program fulfilled all other ACSM criteria for PR in COPD: modality, duration, frequency, and progression.18 Health coaching was done weekly over the telephone to review patient activity and symptoms and to provide an opportunity for the subject to define their weekly goals (Fig. 1).
Program overview.
We randomly assigned subjects at a 1:1 ratio using an online, computer-generated, simple binomial randomization program to 1 of 2 groups (no concealment). There was no blinding of subjects and personnel, nor there was blinding of outcome assessors. Group 1 received the 8-week intervention, and Group 2 had an 8-week control period and then was compassionately offered the intervention after completing the measures at the end of the control period.
Inclusion Criteria
Subjects diagnosed with COPD by a clinician (primary inclusion criteria), confirmed by GOLD guidelines and eligible for PR, age ≥ 40 y, with ≥ 10 pack-years of smoking, and able to speak English were eligible for inclusion. Exclusion criteria were a high likelihood of being lost to follow-up (eg, patients with active chemical dependence), not being able to complete measures, or having severe cognitive impairment (ie, having a higher risk of not completing PR).19 The clinical trial ran from September 2016 through April 2019. The study was approved by the Mayo Clinic institutional review board (#14-009016) and posted in ClinicalTrials.gov (NCT02557178) on September 2015, and the first subject was randomized in September 2016.
The Home-Based System
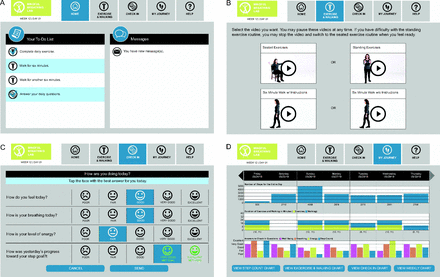
Subjects in the intervention arm received an Android tablet (Google, Menlo Park, California) with the interactive PR program and cellular service (Verizon Communications, New York, New York), a FDA-approved pulse oximeter (3150 WristOx2; Nonin Medical, Plymouth, Minnesota), and a Vívofit activity monitor (Garmin, Schaffhausen, Switzerland) (Fig. 1). Both the pulse oximeter and the activity monitor were connected to the tablet via Bluetooth. The PR program presented a daily to-do list, which offered 4 items (Fig. 2): (1) Complete daily exercises (ie, 11 min of breathing-focused arm movements, either standing or sitting [flexibility practice]), (2) Walk 6 min (ie, a very slow, balance-driven, and breathing-focused in-home walk [balance practice]), (3) Walk for another 6 min, and (4) Answer your daily questions (ie, 3 self-report questions to monitor daily symptoms to track overall well-being, breathing, and energy). Subjects were requested to complete the daily exercise practice (very slow walks) 6 d per week. All activity completed (ie, steps, reporting symptoms, and daily exercises with oxygenation and heart rate data) was visible to the health coach on a secure website (Fig. 2). Activities were designed using the FITT principles of Frequency (ie, 6 d per week), Intensity (ie, low), Time (ie, 20 min), and Type (ie, upper extremity [Arm–Size] and lower extremity [walking]).20
Tablet views. A: Main screen; (B) exercise screen; (C) daily check-in screen; and (D) tablet weekly report (patient view).
The tablet program also offered visualization of progress in a chart tab, where subjects could see auto-populated graphs displaying their steps, exercise minutes, and symptom check-in answers across the 8 weeks of the intervention. A Help tab was also available for frequently asked questions, as well as an area where subjects could type messages to their health coach.
While there was no threshold established a priori for what was considered completion, given the exploratory nature of the study, 6 weeks was considered the minimum dose of home PR.
Health Coaching
Health coaching included weekly calls from health coaches trained in Motivational Interviewing.11,21 The method of training involved a textbook, Building Skills in Motivational Interviewing,22 role-playing, and a simulation program (SIMmersion, Columbia, Maryland) that monitors proficiency in Motivational Interviewing and the number of sessions of practice completed. To assess for fidelity to the Motivational Interviewing techniques, 5% of the calls were reviewed and scored using the global ratings from the Motivational Interviewing Treatment Integrity tool.23
In each call, health coaches reviewed the progress of the subject (Fig. 2D), pointed out trends, and encouraged subject engagement and feedback as it related to their own experience and progress. Full details of the intervention were previously published.2
Measures
All subjects completed questionnaires and 1 week of wearing an ActiGraph wGT3X-BT activity monitor (ActiGraph, Pensacola, Florida) at baseline and after the 8-week intervention or control period. Subjects in the intervention arm completed another set of measures 8 weeks after finishing the home-based program to test the trajectory of outcomes after completion of the intervention. Questionnaires included the Chronic Respiratory Questionnaire–Self-Administered (CRQ-SAS),24 Modified Medical Research Council (mMRC),25 Self-Management Ability Scale (SMAS-30),26 and Working Alliance Inventory–Short Revised (WAI-SR).27 Daily physical activity was measured with the ActiGraph activity monitor, which has been validated and used to assess physical activity in individuals with COPD.28 There were 2 activity monitors used in this study, both worn on the wrist: the Actigraph, which is the accepted standard for physical activity as outcome, and the Garmin monitor that was part of the system for the subject and the coach to set goals of activity in daily life. We used ACSM guidelines for metabolic equivalent of task (MET) cutoff to analyze activity monitor data: light-intensity physical activity is defined as requiring 2.0–2.9 METs, moderate as 3.0–5.9 METs, and vigorous as ≥ 6.0 METs.
Qualitative Interviews and Analysis
Following the intervention, structured qualitative interviews were conducted by telephone with a random sample of subjects who completed the intervention. Responses to scaled items were analyzed using descriptive statistics. Responses to open-ended questions were analyzed using methods of content analysis.29 Two members of the study team (BT and JLR) reviewed transcripts and assigned content to codes representing key domains of the survey (eg, technology satisfaction), as well as codes that represented latent content (eg, emotional responses to intervention participation). Computer-assisted qualitative data analysis software (NVivo 12, QSR International, Doncaster, Australia) was used to facilitate data organization and queries. Qualitative data were explored by respondent outcomes (eg, dichotomized variables based on a significant change in SMAS-30 total and CRQ scores) to understand potential variation in experiences.
Mixed Methods Analysis
This study employed a convergent approach to mixed methods, in which data from questionnaires and interviews were first analyzed separately and then compared to more fully understand program effectiveness.30 Members of the study team met to review and discuss findings from each method, including those on similar constructs (eg, improvements in dyspnea and self-management); side-by-side comparison aided in interpretation. Methodological and analyst triangulation served to minimize interpretive bias and increase the credibility of findings.31
Statistical Analysis
Baseline subject characteristics were compared between study arms and tested for significance using chi-square tests for categorical variables, t tests for normally distributed continuous variables, and appropriate nonparametric tests for non-normally distributed continuous variables. Primary outcomes were adherence and CRQ Dyspnea. Secondary outcomes were self-management measured with the SMAS-30 and the remaining CRQ domains.
Outcomes were compared by group assignment using generalized linear models with a normal distribution with identity link for continuous outcomes, Poisson distribution with log link for count outcomes, and binomial distribution with logit link for binary outcomes. Hypothesis tests were 2-sided, with P values < .05 considered statistically significant. A robust standard error was used to accommodate missing data under the assumption that the outcomes were missing at random. The target sample size of 128 subjects provided approximately 80% power to detect 0.5 points (considered the minimal clinically important difference) in the CRQ-SF Dyspnea domain score. We assumed 20% attrition, and a final sample of 154 subjects was proposed. A trajectory analysis looked at changes from baseline to follow-up at weeks 9 and 17 in the intervention group to investigate if the change seen after the intervention were maintained after 8 weeks postintervention. One-sample, 2-sided t tests, with 5% type 1 error rates, were used to determine whether these changes were significantly different from zero.
Results
A total of 154 subjects signed consent to participate between December 2016 and March 2018. Subjects were randomized to control (n = 76) and intervention (n = 78) groups (Fig. 3). A total of 119 (77%) subjects completed the study. There were no significant differences in reasons for dropout by arm. Demographics and baseline measures are shown in Table 1.
Flow chart. CRQ = Chronic Respiratory Questionnaire.
Baseline Clinical and Demographic Characteristics of Subjects
The adherence to the program (expressed in the percentage of total prescribed days) was 86% adherence for the prescribed days of the system use (≥ 1 feature), 80% for the balance-related walking practice, and 87% for the flexibility-related seated or standing exercises. Subjects’ adherence to using the Garmin monitor was 55% or 33 d out of the 60-d intervention. From a safety perspective, analytics from the system indicated that the videos were watched and practiced (eg, having heart rate and oxygen saturation data) > 6,000 times without reported adverse effects (ie, falls, physical injury, symptoms of shortness of breath or exacerbations).
Table 2 shows an overall comparison of outcomes between intervention and control subjects (ie, changes from baseline to Week 9). There was a trend (P = .062) but no significant difference in the primary clinical outcome of the study (CRQ Dyspnea). Self-management total score and the Self-management domains of Taking Initiative and Investment Behavior were statistically improved after the intervention (P < .001, .01 and < .001, respectively). There was no improvement in physical activity.
Comparison of Intervention and Control Subject Changes From Baseline to Week 9
Subjects who did not drop out received an average of 6 calls during the 8-week intervention. Both groups reported feeling comfortable (> 5 on a self-efficacy scale of 1–10) using a smartphone or a computer (control 79%, intervention 83%), as a measure of confidence to use technology.
Trajectory Analysis
In this within-group analysis (ie, intervention subjects only), the CRQ domains of Dyspnea and Mastery exhibited a significant change from baseline, and the CRQ domain of Emotion became significant at 8 weeks after the intervention finished. SMAS-30 Total and the Domain Investment Behavior remained significant (P < .05 for all) (Table 3). Physical activity was not significantly changed.
Trajectory Analysis in Intervention Subjects Only
Engagement With the Health Coach
With regard to the WAI-SR score, the Task domain, the Bond domain, and the Goal domain were ≥ 80% of the maximum possible scores, indicating high engagement between the subject and health coach. In an exploratory analysis we found the Task domain in the WAI-SR to be associated with the improvement in SMAS-30 total after adjusting for age, gender, FEV1, and MMRC score (P = .01, model not shown). Twenty-eight participants completed telephone interviews.
Interviews
Interview respondents were not significantly different from the overall study subjects in terms of age, gender, FEV1%, or mMRC Dyspnea. Results of the scaled interview questions indicate high levels of acceptability and satisfaction with the program. Subjects stated that the individual components were easy to use, and 96% said they would recommend the program to others. The vast majority of subjects also rated the coaching component highly in terms of how well the coach listened and how much coaching increased their confidence (Table 4).
Scaled Interview Questions
Results of the analysis of open-ended question responses complemented these results and, in some cases, helped explain them. In terms of program satisfaction, subjects liked the technology and the ability to see their progress (Table 5).
Qualitative Feedback
Qualitative analysis related to the experience with health coaching revealed 2 views on the benefits of coaching in this program. The first was that coaching provided subjects with a range of emotional or psychological benefits, including a sense of encouragement, reassurance, and understanding. Subjects felt supported by a coach who was interested in how they were doing. Several subjects stated that they wished the program was longer because they reported value in the coaching relationship and the accountability it provided (Table 5).
The second view was that coaching was beneficial because the coach's informative support helped subjects be more effective in their technology use, including troubleshooting technology issues so they could fully engage with the technology components, as well as the education that the coach provided about the disease and reasons for program components (Table 5).
Finally, in terms of program effectiveness, a majority of subjects reported that they felt the program was effective because they perceived their breathing was improved. This sense of physical improvement was a reason they would recommend the program to others. Similarly, some subjects described the effectiveness of a holistic approach (ie, combining technology and coaching) for people with COPD. One subject described how this approach improved breathing and provided psychological benefits (Table 5).
Mixed Methods Integration
Placing the results of the quantitative and qualitative results side by side (ie, quantitative primary outcomes dichotomized into half standard deviation improvement or not), the study team identified several ways in which the triangulation of data sources and methods provided a fuller picture of the program experience and outcomes. These fall under the categories of (1) self-management and self-efficacy, and (2) dyspnea and quality of life.
In terms of self-management and self-efficacy, the qualitative data provided insights into how the technology and the coaching worked together to keep subjects engaged in the exercises and learning how to feel good about managing their COPD with appropriate techniques. In terms of dyspnea, qualitative analysis of interview data, dichotomized by improvement in the dyspnea measure, showed that subjects felt like their breathing improved, regardless of whether the measure showed significant improvement in CRQ Dyspnea (> 0.5 points). Subjects’ self-described sense of improved breathing was tied to comments stating that the program was effective and worthwhile.
Discussion
We explored the feasibility uptake and adherence of a home-based rehabilitation program with health coaching in patients with COPD. We found 86% adherence for the prescribed days of the system use, 80% adherence to the walking practice, and 87% adherence to the seated or standing flexibility exercises that was prescribed to be done 6 d per week. This level of adherence is in itself a significant result, considering prior evidence that shows 50% of patients with COPD decline to participate in PR and 30–50% drop out before completion.3,5,8 Our adherence results that seem higher than the reported center-based PR suggest that a program with a lower intensity, done at home with collaborative support from a health coach, is well accepted. This finding alone in this study is worth being reported and further explored, and it may increase the implementation and dissemination of rehabilitation (which, at its core, is intended to restore function) to individuals with COPD.
The study was probably underpowered to achieve statistically significant improvement in the primary outcome of CRQ Dyspnea (breathlessness) when compared to an active control group (P = .062). However, we found a significant improvement in CRQ Dyspnea in a within-group analysis was improved at 8 weeks and that improvement was maintained at 17 weeks (8 weeks after finishing the intervention) (P = .01) (Table 3). The CRQ Mastery domain also improved significantly after the intervention (P = .047) and was maintained at 17 weeks (P < .001) in this within-group analysis, further supporting the significant improvement in self-management abilities that we believe is clinically important and worth reporting.
Self-management, one of the secondary outcomes proposed, was measured with the SMAS-30 total score and improved significantly after the home-based intervention. In particular, we noted improvement in the domains Taking Initiative and Investment Behavior. The latter provides a novel mechanistic understanding of the effect of the home-based intervention on self-management abilities. The Taking Initiative domain is in line with self-efficacy beliefs and adds the individual's power to control his or her goals and next steps. This domain reflects a proactive and self-reflecting ability that empowers taking action. The Investment Behavior domain refers to the ability to dedicate time and action planning to achieve stability and maintenance of resources for the long-term management of chronic illness. It relates to the uncertainty of how life may unfold and that Investment Behavior may help future well-being. Our findings on Taking Initiative and Investment Behavior domains not only provide insights on the effect of the intervention on self-management abilities but also may represent targets for self-management interventions or health coaching in COPD. Our findings confirm and extend previous results on empowerment and increased self-efficacy after home-based interventions.32-34
Health coaching in this study may have been a key element in promoting self-efficacy in managing COPD and was added with that very purpose: to increase self-management abilities consistent with our previous findings in COPD subjects.35,36
Subject Engagement During Heath Coaching
The WAI-SR mean total scores were high, with 85% of maximum scores indicating a high degree of engagement between the health coach and the subjects. Our results further suggest that the engagement between the subjects and the health coach expressed in the WAI-SR is associated with clinical outcomes; ie, the improvement of the task domain of the WAI-SR was associated with improvement of fatigue, the second most important outcome in COPD.37 Previous data suggest that a therapeutic alliance correlates with positive treatment outcomes38,39; the alliance between the patient and the health coach can be measured and appears to be strongly associated with the patient’s adherence to and satisfaction with treatment.40 A review by Martin et al41 concluded that the overall relation of the therapeutic alliance with the outcome is moderate but consistent. Measuring a working alliance may represent a desirable outcome to measure in-home health coaching programs.
Our qualitative analysis found high subject acceptability with this program, which is consistent with the adherence rates that we observed. An important aspect of the program is that we were able to document objective compliance by reviewing the sessions captured by the system, which provided an accurate assessment of compliance to the intervention in contrast to self-reporting, which can have a significant bias.
The intervention lacked improvement in daily physical activity, a very significant knowledge gap in COPD as it is unclear in currently available evidence how to improve physical activity in individuals with COPD.42 Further, we acknowledge that the intensity of the program was not as recommended by guidelines; however, we believe that, for unsupervised home-based programs, like the one tested, new guidelines are needed to ensure the safety of participants. We feel that our results inform the current understanding of home-based programs, particularly in view of the high adherence and improvement in self-management abilities.
Results from our qualitative study on subjects who completed the intervention suggest their preference for a longer intervention to increase the chance of making a difference in physical activity. Our analytic results on safety (ie, no falls or adverse events) are novel and important; this is the first report of this magnitude (> 6,000 documented sessions) on safety for a home-based program. The lack of a significant effect on CRQ Dyspnea (primary outcome) after the program ended may suggest an ineffective intervention; however, we achieved a trend toward statistical significance (P = .06), and, in our qualitative-quantitative triangulation, subjects reflected a sense of improvement in breathlessness despite the lack of a rigid statistically significant improvement in CRQ Dyspnea. Subjects also reported the need for a longer program that may have had a stronger effect on the quality of life outcomes after the intervention.
The implementation aspect of this project may be significant. The system used is compliant with billing for remote patient monitoring ICD codes (CMS CPT 99453, 99454, 99457, and 99458), which would make the program billable, once it is proved to be efficacious.
Limitations
This study has a number of limitations. First, we did not measure exercise capacity. We intentionally planned an intervention that may not have the subjects come to the medical center; we obtained all measures by mail (ie, questionnaires and activity monitors), which helped tremendously with compliance and acceptance to participate. We also found this approach useful in implementing this research intervention during the COVID-19 pandemic. However, future research should consider remote assessments of functional capacity, particularly during the COVID-19 era.43 The lack of health care utilization outcomes is also a limitation of this feasibility study. These elements are being tested in a current research project (RO1 HL 140486, ClinicalTrials.gov registration NCT03480386).
In addition, we are unable to define the contribution of each component of the intervention (ie, health coaching vs home-based rehabilitation with technology) to the improvements observed. However, we hypothesize that both components had additive, if not synergistic, effects. We also cannot affirm the strength of the positive findings, either maintenance of benefits or improvements observed in the trajectory analysis in the intervention group only, 8 weeks after finishing the home-based PR with health coaching intervention as we did not compare to the control group that was compassionately offered the intervention after the end of the control period. We also did not conduct the qualitative analysis with subjects who withdrew during the intervention, so we may have missed additional feedback.
We acknowledge that the testing period may have been not long enough, which may account for the modest results reported. Subjects interviewed in the qualitative study stated the willingness for a longer program, which may have had a stronger effect on the outcomes after the intervention. We are now testing a longer 12-week intervention, which has greater focus on adjusting weekly steps goals based on recent evidence of significant improvement in physical activity based on semi-automated adjustment of weekly step goals.44 Finally, we did not have subjects with mild COPD in this group, however those are not the patients that are prevalent in rehabilitation programs as they are minimally limited.
Conclusions
We believe that this mixed-methods feasibility study represents a step forward in our understanding of home-based programs in COPD patients and may represent the scientific foundation for the design of future home-based interventions. Our results demonstrate the feasibility and high adherence to the tested home-based program with health coaching intervention that improved self-management abilities and may improve dyspnea and overall quality of life. We provide valuable mechanistic information on aspects of self-management that may help fill the knowledge gap in the science of behavior change in COPD and home-based self-management programs.
Footnotes
- Correspondence: Roberto P Benzo MD MSc, 200 First St SW, Rochester, MN 55905. E-mail: benzo.roberto{at}mayo.edu
Supplementary material related to this paper is available at http://www.rcjournal.com.
Dr RP Benzo is supported in part by grants R61 HL142933, K24 HL138150, HL140486 from the National Heart Blood and Lung Institute, National Institutes of Health; and Dr RP Benzo and Ms Seifert are supported in part by grant SBIR HL114162-02A1. The authors have no conflicts to disclose.
- Copyright © 2021 by Daedalus Enterprises