Abstract
BACKGROUND: Patients who are obese are at risk for developing high pleural pressure, which leads to alveolar collapse. Esophageal pressure (Pes) can be used as a surrogate for pleural pressure and can be used to guide PEEP titration. Although recent clinical data on Pes-guided PEEP has shown no benefit, its utility in the subgroup of patients who are obese has not been studied.
METHODS: The Medical Information Mart for Intensive Care-III critical care database was queried to gather data on Pes in subjects on mechanical ventilation. Pes in obese and non-obese groups were compared, and a subgroup analysis was performed in subjects with class III obesity. Thereafter, empirical and Pes-guided PEEP protocols of a recently published trial were theoretically applied to the obese group and ventilator outcomes were compared.
RESULTS: A total of 105 subjects were included in the study. The average end-expiratory Pes in the obese group was 18.8 ± 5 cm H2O compared with 16.8 ± 4.8 cm H2O in the non-obese group (P < .05). If Pes-guided PEEP protocol was to be applied to those in the obese group, then the PEEP setting would be significantly higher than empirical PEEP setting. These findings were accentuated in the subgroup of subjects with class III obesity.
CONCLUSIONS: Individualization of PEEP with Pes guidance may have a role in patients who are obese.
Introduction
Patients who are obese and on mechanical ventilation have a propensity to develop high pleural pressures due to mass loading of the chest wall.1-3 This occurs due to the excessive weight of adipose tissue on the thoracic cage as well as the effect of increased abdominal pressure on the diaphragm. High pleural pressures reduce the end-expiratory transpulmonary pressure. Transpulmonary pressure is the difference between airway pressure and pleural pressure. In the absence of airway closure and air flow, transpulmonary pressure reflects the elastic recoil pressure of the lung. Although a simplification, end-expiratory transpulmonary pressure needs to be zero or positive to maintain alveolar patency. Esophageal pressure (Pes), a surrogate of pleural pressure, could be used to individualize PEEP titration. The recently published EPVent-2 trial4 failed to show a clinical benefit of routine utilization of Pes-guided PEEP in subjects with ARDS. In this trial, Pes-guided PEEP was compared with high empirical PEEP in all participants with moderate to severe ARDS.4
Patients with high pleural pressures may still benefit from Pes guidance. The key rationale behind this idea is that the presence of higher-than-average Pes at end expiration would result in a lower end-expiratory transpulmonary pressure for the same level of PEEP. Hence, even high empirical PEEP may not produce adequate end-expiratory transpulmonary pressure in patients with high end-expiratory Pes. The purpose of this study was to perform a retrospective analysis of Pes in subjects who were obese and on mechanical ventilation, and to compare them with subjects who were not obese. The objective was to evaluate the potential impact of the application of the EPVent-2 trial4 Pes-guided PEEP protocol in subjects who were obese. We hypothesized that the use of Pes guidance would result in a higher PEEP setting in patients who were obese compared with empirical PEEP.
QUICK LOOK
Current Knowledge
Esophageal pressure guidance is a physiologically appealing method of PEEP pressure titration. However, a recently published trial found no clinical benefit of routine utilization of esophageal pressure-guided PEEP in subjects with ARDS. It remains unclear if selective utilization of esophageal pressure guidance may still be beneficial.
What This Paper Contributes to Our Knowledge
Obese subjects demonstrated a higher end-expiratory esophageal pressure (Pes) than non-obese subjects. PEEP guided by Pes gudiance to acheive a positive transpulmonary pressure would have resulted in much higher PEEP than empirically derived PEEP. These effects were more pronounced in subjects with class III obesity.
Methods
The Medical Information Mart for Intensive Care-III critical care database was queried for this analysis.5 This is an openly available database composed of 53,423 distinct hospital admissions of adult patients admitted to critical care units of a tertiary care hospital between 2001 and 2012. Because this is an openly available de-identified database, this study was exempt from institutional board review approval. The information on the specific protocol used for obtaining Pes was not available from the database. The study included all patients in whom end-expiratory transpulmonary pressure and total PEEP were charted concurrently. In cases in which more than one set of values were available, the earliest set was included for the analysis. End-expiratory Pes was calculated by subtracting end-expiratory transpulmonary pressure from total PEEP because direct documentation of Pes was not available from the database. The following baseline characteristics were recorded: age, sex, body mass index (BMI), and  .
.
For the primary analysis, the average end-expiratory Pes was compared between the subjects who were obese (BMI ≥ 30 kg/m2) and those who were not obese (BMI < 30 kg/m2). A subgroup analysis was also performed on the subjects with class III obesity (BMI > 40 kg/m2). Thereafter, the Pes-guided PEEP and empirical PEEP protocols from the EPVent-2 trial4 were theoretically applied to all the subjects with obesity as well as those with class III obesity (Fig. 1). The idea behind doing this was to project how these 2 protocols would affect the PEEP setting (and, therefore, end-expiratory transpulmonary pressure) based on the prevailing Pes and  values of the study population. For a given value of
values of the study population. For a given value of  , the lowest PEEP setting that corresponded to that value was used from the respective PEEP protocol of the EPVent-2 trial.4
, the lowest PEEP setting that corresponded to that value was used from the respective PEEP protocol of the EPVent-2 trial.4
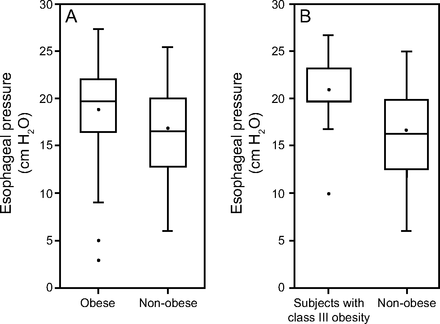
Box plots that compare esophageal pressures in the non-obese versus obese group as well as subjects who were not obese versus subjects with class III obesity. The upper and lower limit of the box represent third quartile (Q3) and lower quartile (Q1) respectively. The horizontal line within the box represents median. (Note that median of the first box in panel B happens to be equal to Q3). Points inside of boxes denote the meanwhile upper and lower whiskers represent maximum and minimum values, excluding outliers. Points outside of boxes represent outliers.
Statistical Analysis
Collected data were summarized as mean ± SD for all normally distributed continuous variables and as median (interquartile range [IQR]) for non-normally distributed continuous variables. The 2-sample t test was used to compare normally distributed continuous variables. The Kruskal-Wallis test was performed to compare non-normally distributed continuous variables. All the analyses were performed by using the SAS 9.4 for Linux (SAS, Cary, North Carolina). The level of statistical significance was set at P < .05 (2-tailed).
Results
A total of 105 subjects were eventually included for the analysis. The baseline characteristics of the patient population are summarized in Table 1. The median (IQR) BMI of the non-obese group was 26.6 (24–28.1) kg/m2 and that of the obese group was 35.9 (32.9–44.2) kg/m2. There was a positive correlation between BMI and end-expiratory Pes (Pearson r = 0.29; P = .002). The median (IQR) timing of the Pes measurement for the study population was 3 (1–7) d after admission. The difference between the time from admission to Pes measurement was not statistically significant in either group (P = .83). 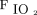 at the time of analysis was 73 ± 20% in the non-obese group and 70 ± 21% in the obese group. The in-hospital mortality rate of the study cohort was 39% (41/105).
at the time of analysis was 73 ± 20% in the non-obese group and 70 ± 21% in the obese group. The in-hospital mortality rate of the study cohort was 39% (41/105).
Baseline Characteristics of the Study Population
Primary Analysis: Comparison of Non-Obese versus Obese
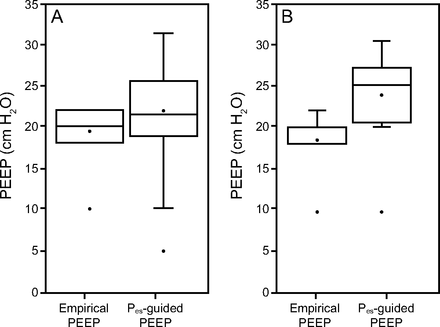
The average end-expiratory Pes in the obese group was 18.8 ± 5 cm H2O compared with 16.8 ± 4.8 cm H2O in the non-obese group (P = .04) (Tables 2 and 3). The difference between the in-hospital mortality of the obese versus non-obese groups did not reach statistical significance (32.8% vs 47.7%; P = .12). On theoretical application of the EPVent-2 trial4 PEEP protocols to the obese group, the average Pes-guided PEEP would be 21.7 ± 5.2 cm H2O compared with the average empirical PEEP of 19.4 ± 3.2 cm H2O (P = .004). Furthermore, with the use of the Pes-guided protocol, the average end-expiratory transpulmonary pressure in these subjects who were obese would be 2.8 ± 2.3 cm H2O compared with 0.5 ± 6.2 cm H2O without Pes guidance (P < .001). Importantly, end-expiratory transpulmonary pressure would be negative in 44% (27/61) of these subjects with the empirical PEEP protocol.
Comparison of Subjects Who Were Obese vs Subjects Who Were Not Obese
Application of EPVent-2 PEEP Protocols in Subjects Who Were Obese
Subgroup Analysis: Comparison of Non-Obese versus Subjects with Class III Obesity
When comparing the subjects with class III obesity versus the non-obese group, the difference in the average end-expiratory Pes was higher (21.3 ± 3.8 cm H2O vs 16.8 ± 4.8 cm H2O; P < .001) (Tables 4 and 5); the in-hospital mortality in the 2 groups was 30% versus 48% (P = .19). On hypothetical application, the EPVent-2 trial4 PEEP protocols in this subgroup, the mean ± SD difference between Pes guided PEEP and empirical PEEP would be even larger than when applied to the whole obese group (23.7 ± 4.5 cm H2O vs 18.5 ± 3.9 cm H2O; P < .001). Also, the mean ± SD end-expiratory transpulmonary pressure with Pes guidance would be much higher than the mean ± SD end-expiratory transpulmonary pressure without Pes guidance in this subgroup (2.2 ± 2.27 cm H2O vs –2.8 ± 4.87 cm H2O; P < .001). Also, the end-expiratory transpulmonary pressure would be negative in 65% (13/20) of these subjects with the empirical PEEP protocol. Pictorial representation of the data are presented in Figures 2 and 3.
Comparison of Subjects with Class III Obesity vs Subjects Who Were Not Obese
Application of EPVent-2 PEEP Protocols in a Subgroup of Subjects with Class III Obesity
Box plots that compare theoretical application of the 2 EPVent-2 trial4 PEEP protocols in A: all obese subjects and B: subjects with class III obesity. The upper and lower limit of the box represent third quartile (Q3) and lower quartile (Q1) respectively. The horizontal line within the box represents median. (Note that median of the first box in panel B happens to be equal to Q3). Points inside of boxes denote the meanwhile upper and lower whiskers represent maximum and minimum values, excluding outliers. Points outside of boxes represent outliers.
Flow chart delineating how the post-hoc analysis was performed in the obese group of the study population using the EPVent-2 PEEP protocols. End-expiratory transpulmonary pressure was also compared, and comparisons were subsequently performed in the subgroup of subjects with class III obesity.
Discussion
The results of this study showed that end-expiratory Pes was significantly higher in the subjects who were obese compared with the subjects who were not obese. By using the PEEP strategies of the EPVent-2 trial,4 Pes guidance would result in significantly higher PEEP and end-expiratory transpulmonary pressure values in the obese group. These differences were accentuated further when the non-obese group was compared with the subjects with class III obesity. Respiratory mechanics are significantly altered in patients who are obese. In recumbent patients who are obese, increased fat within the chest wall causes mass loading of the respiratory system, which thereby leads to increased pleural pressures.1,2 Behazin et al1 demonstrated this in the subjects who were obese and without lung disease. In this study, Pes was found to have a good correlation with another estimate of pleural pressure derived from airway pressure and flow.1 In another study, on healthy subjects, Pes during supine position was found to be significantly higher than in the sitting position in both subjects who were obese and those who were lean.6 Patients who are obese and are on mechanical ventilation for respiratory failure are another important population to consider. In a recent study that enrolled 15 subjects, Pes was found to be higher in the subjects who were obese compared with those who were not obese.7 Yet, there remains a paucity of data exploring this issue.
The pressure across a distensible chamber (transmural pressure) is the true distending pressure of the chamber. From the perspective of a single alveolus, this would be the transalveolar pressure (alveolar pressure minus pleural pressure). The distending pressure across the entire lung parenchyma has traditionally been referred to as “elastic recoil pressure of the lung.” As alluded to before, transpulmonary pressure reflects elastic recoil pressure of the lung in the absence of air flow and airway closure. This explains why end-inspiratory transpulmonary pressure and end-expiratory transpulmonary pressure are better markers of lung parenchymal stress than plateau pressure and PEEP, respectively.
The considerations discussed thus far are even more pertinent in patients with class III obesity, as reflected in our analysis. Although the average end-expiratory Pes of the obese group as a whole was higher than that of the non-obese group, it was higher still in the subgroup of subjects with class III obesity. It is notable that, even in subjects who were not obese, the average end-expiratory Pes was 16.8 ± 4.8 cm H2O, which is higher than that observed in healthy non-obese subjects.6 Similar values have been noted in other clinical studies.8 The average end-expiratory Pes in the 2 groups of the EPVent-2 trial4 were 15 cm H2O and 16 cm H2O, respectively. Various explanations have been proposed for this peculiar observation, including the presence of intra-abdominal hypertension, which is widely prevalent in patients who are critically ill.9,10 Furthermore, the gravitational pleural pressure gradient is accentuated in patients with ARDS due to excessive weight of the edematous lung tissue. This causes a further increase in pleural pressure of dependent lung regions, which is more closely recorded by the esophageal balloon.
Determining the optimal PEEP remains a challenge, especially in patients who are obese and on mechanical ventilation. Pes can help individualize PEEP selection by identifying the minimum value of PEEP required to maintain a positive end-expiratory transpulmonary pressure. Large deviations (positive or negative) of set PEEP from end-expiratory Pes increases the risk of lung injury from volutrauma or atelectrauma.11 Volutrauma refers to mechanical injury caused by over-distention of lung units. This can occur if set PEEP is significantly higher than end-expiratory Pes. Alternatively, if the PEEP is set much lower than the end-expiratory Pes, the resulting negative end-expiratory transpulmonary pressure may cause expiratory collapse of lung units, especially in patients with ARDS who have surfactant dysfunction. Delivery of a positive-pressure breath during inspiration may then lead to opening of the atelectatic lung regions. This cyclical collapse and re-opening of lung units causes atelectrauma and worsens lung injury.
More commonly, PEEP is set empirically based on the prevailing  value. The rationale for this approach is that higher
value. The rationale for this approach is that higher  requirements are suggestive of an intrapulmonary shunt due to atelectatic lung units that can be recruited by applying higher PEEP. For example, the commonly used empirical PEEP protocol is used in the ARDSNet study of the National Heart, Lung, and Blood Institute correlates
requirements are suggestive of an intrapulmonary shunt due to atelectatic lung units that can be recruited by applying higher PEEP. For example, the commonly used empirical PEEP protocol is used in the ARDSNet study of the National Heart, Lung, and Blood Institute correlates 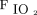 and PEEP.12 Recently, a more aggressive empirical PEEP strategy to maximize lung recruitment has been tested. This open lung strategy that uses a higher PEEP has been found to improve lung function and mechanics.13 A signal of reduced mortality in the subgroup of patients with ARDS was also noted in a meta-analysis.14
and PEEP.12 Recently, a more aggressive empirical PEEP strategy to maximize lung recruitment has been tested. This open lung strategy that uses a higher PEEP has been found to improve lung function and mechanics.13 A signal of reduced mortality in the subgroup of patients with ARDS was also noted in a meta-analysis.14
The EPVent was a pilot study that compared Pes-guided PEEP to standard empirical PEEP in subjects with ARDS by using the PEEP table from the ARDSNet study.15 That study showed considerable improvements in oxygenation and lung compliance with Pes-guided PEEP. Notably, the set PEEP and end-expiratory transpulmonary pressure were significantly higher in the Pes-guided group, which indicated that the empirical PEEP group may have received a lower than optimal PEEP. A larger subsequent study, named EPVent-2,4 had a higher empirical PEEP in the control arm for comparison with Pes-guided PEEP. This choice for the control arm was also influenced by other preliminary evidence that suggests benefits with higher empirical PEEP.13,14 Interestingly, no difference in the PEEP and end-expiratory transpulmonary pressure pressure was seen in the 2 groups of EPVent-2.4 There also were no differences in clinical outcomes with Pes guidance, including mortality and days free from mechanical ventilation.
Despite the negative results of the EPVent-2 trial,4 it can be argued that Pes guidance may be of value as in patients with higher-than-average pleural pressures. Obesity is an important risk factor for this, as demonstrated in our study. Intra-abdominal hypertension may also result in elevated pleural pressures. In these patients, the high end-expiratory Pes may result in lower than optimal PEEP setting with the empirical strategy. We tested this hypothesis in the obese group of our study population by theoretical application of the 2 PEEP protocols of the EPVent-2 trial.4 In line with the finding of higher values of Pes in this group, it was found that Pes guidance would result in significantly higher PEEP and end-expiratory transpulmonary pressure in these subjects. Because Pes-guided PEEP is considered a surrogate for optimal PEEP, it can be extrapolated that empirical PEEP may result in suboptimal PEEP in patients who are obese. In other words, reliance on empirical PEEP may lead to a higher incidence of negative end-expiratory transpulmonary pressure, thereby promoting atelectasis and atelectrauma.
Although our study found a statistically significant difference between the subjects who were obese and those who were not obese for empirical PEEP versus Pes-guided PEEP, the difference was modest (2.3 cm H2O) and may not be clinically important. However, a few considerations should be made. First, the difference in PEEP was higher in subjects with class III obesity. Perhaps the higher the BMI, the more likely that Pes-guided PEEP would be higher than empirically set PEEP. Second, the major advantage of Pes-guided PEEP approach is the ability to individualize it to improve outcomes. For instance, in a subgroup analysis of the EPVent-2 trial,4 the subjects in the control group in whom end-expiratory transpulmonary pressure was ±2 cm H2O were found to have a higher survival.16
In the subgroup of subjects with class III obesity, we found that the effects of Pes guidance would be magnified. This was reflected in the larger differences between the expected PEEP and end-expiratory transpulmonary pressure. Furthermore, the average end-expiratory transpulmonary pressure would be negative (–2.8 ± 4.9 cm H2O; range –17 to 3 cm H2O). Because a higher empirical PEEP was used for these analyses, we speculated that use of the standard empirical PEEP would amplify these differences. Another important feature of empirical PEEP is its ceiling value. In both standard and high empirical protocols, the maximum allowed value of PEEP was 24 cm H2O. In our study population, 4.9% (2/41) of the subjects who were not obese, 13.1% (8/61) of the subjects who were obese, and 20% (4/20) of the subjects with class III obesity had a Pes of >24 cm H2O. Empirical PEEP will always result in a negative end-expiratory transpulmonary pressure in these patients, regardless of the current  requirement.
requirement.
Major limitations of our study included its retrospective nature and the inclusion of an unselected patient population. Technical details of Pes measurements were not available from the Medical Information Mart for Intensive Care-III critical care database. Despite the large size of the database, the number of subjects included in the study was small. This was likely because Pes measurement is not routinely performed in patients on mechanical ventilation. We are unable to make any remarks on whether Pes guidance would improve outcomes in patients who are obese and on mechanical ventilation with or without ARDS. However, we suggest that these patients are at a significant risk for developing negative end-expiratory transpulmonary pressure even with a higher empirical PEEP, the implications of which would be greater in the presence of ARDS. Apart from obesity, there are other physiologic features that lead to high pleural pressures, for example, significant intra-abdominal hypertension.17
Conclusions
In this analysis, end-expiratory Pes was significantly higher in subjects who were obese and on mechanical ventilation compared with subjects who were not obese. In a post hoc analysis, we found that Pes guidance when using the EPVent-2 trial4 protocol would result in a significantly higher PEEP setting than when using high empirical PEEP. This population would be at risk for developing negative end-expiratory transpulmonary pressures, even with high empirical PEEP. Hence, Pes guidance may lead to better optimization of PEEP in patients at risk for having high pleural pressures. This concept can be tested in future clinical studies.
Footnotes
- Correspondence: Guramrinder Singh Thind MD, Department of Critical Care Medicine, Respiratory Institute, Cleveland Clinic, 9500 Euclid Ave, Cleveland, OH 44915. E-mail: thindg{at}ccf.org
Mr Chatburn discloses relationships with IngMar Medical, Vyaire Medical, Inovytec, and Promedic LLC. The other authors have disclosed no conflicts of interest.
- Copyright © 2022 by Daedalus Enterprises