Abstract
BACKGROUND: Despite expert recommendations for use, limited evidence identifies effectiveness of mechanical insufflation-exsufflation (MI-E) in addressing respiratory morbidity and resultant health care utilization and costs for individuals with neuromuscular disorders. We examined the impact of provision of publicly funded MI-E devices on health care utilization, health care costs, and survival trajectory.
METHODS: This is a retrospective pre/post cohort study linking data on prospectively recruited participants using MI-E to health administrative databases to quantify outcomes.
RESULTS: We linked data from 106 participants (8 age < 15 y) and determined annualized health care use pre/post device. We found no difference in emergency department (ED) visit or hospital admission rates. Following MI-E approval, participants required fewer hospital days (median [interquartile range] [IQR]) 0 [0–9] vs 0 [0–4], P = .03). Rates of physician specialist visits also decreased (median IQR 7 [4–11] vs 4 [2–7], P < .001). Conversely, rates of home care nursing and homemaking/personal support visits increased. Following MI-E, total costs were lower for 59.4%, not different for 13.2%, and higher for 27.4%. Physician billing costs decreased whereas home care costs increased. Regression modeling identified pre-MI-E costs were the most important predictor of costs after approval. At 12 months, 23 (21.7%) participants had died. Risk of death was higher for those using more medical devices (hazard ratio 1.12, [95% CI 1.02–1.22]) in the home.
CONCLUSIONS: Provision of publicly funded MI-E devices did not influence rates of ED visits or hospital admission but did shift health care utilization and costs from the acute care to community sector. Although increased community costs negated cost savings from physician billings, evidence suggests costs savings from reduced hospital days and fewer specialist visits. Risk of death was highest in individuals requiring multiple medical technologies.
Introduction
Mechanical insufflation-exsufflation (MI-E) devices aid in secretion clearance and cough by increasing expiratory air flow through application of positive pressure followed by a rapid shift to negative pressure.1 The attendant increase in cough peak flow helps move secretions from distal to central airways. MI-E devices are prescribed for use in the home for individuals with neuromuscular disorders based on the frequency of respiratory infection and declining cough peak flow or percentage of FVC. Cough peak flow declines due to loss of respiratory muscle strength as chest wall muscles become shorter and stiffer.2 Despite being an expensive treatment compared to other airway clearance techniques, and requiring more training at treatment initiation, MI-E with a manually assisted cough was recommended by an expert panel for adults (and children in whom cough peak flow can be measured) once cough peak flow declines below 160 L/min.3
Notwithstanding expert recommendations for use,3 and the known complications of ineffective cough and decreased ventilation in individuals with neuromuscular disorders,4 empirical evidence on the effectiveness of MI-E is limited.5 A 2017 scoping review6 identified 12 studies (4 randomized controlled trials, 3 comparative, and 5 observational studies) enrolling 325 participants in total. Only 2 studies (21 participants) reported health care utilization outcomes; 4 studies reported on subjective symptoms or quality-of-life measures; none reported on survival trajectory.
In the Canadian province of Ontario (population circa 14 million), advocacy work by several organizations including Muscular Dystrophy Canada led to the establishment in April 2014 of the publicly funded Provincial Cough Assist Program by the Ministry of Health and Long-Term Care. The program, managed by the Ontario Ventilator Equipment Pool, provides publicly funded MI-E devices for use at home by individuals with neuromuscular respiratory insufficiency. Devices are prescribed by approved respiratory specialist physicians based on the following criteria: (1) cough peak flow < 270 L/min measured using lung volume recruitment or manually assisted cough; (2) any neuromuscular disease, post polio, spinal cord injury, or weak respiratory muscles or paralysis; and (3) at risk of or ventilator assisted. Individuals receive an initial 1–2 h training session on MI-E but no other specific support in the home for its ongoing use.7 Prior to April 2014, individuals had to purchase their own device (or use private insurance) at a cost of > US $5,460. Since the program’s inception, the demand for MI-E devices has been well above the predicted need for these devices in the community. However, the rollout of the program did not include strategies to evaluate its effect on health system utilization, costs, or survival trajectory.
Therefore, our objective was to examine the impact of provision of publicly funded MI-E devices for use in the home on (1) hospital (eg, emergency department [ED] visits, hospital admissions) and community (eg, home health care) health service utilization, (2) health care costs, and (3) survival trajectory.
QUICK LOOK
Current Knowledge
Mechanical insufflation-exsufflation (MI-E) with a manually assisted cough was recommended by an expert panel for adults (and children in whom cough peak flow can be measured) once cough peak flow declines below 160 L/min. Despite these expert recommendations for use and the known complications of ineffective cough and decreased ventilation in individuals with neuromuscular disorders, empirical evidence on the effectiveness of MI-E is limited.
What This Paper Contributes to Our Knowledge
This study reports real-world health care utilization and cost data on to date the largest prospectively recruited cohort of individuals with a neuromuscular disorder using MI-E in the home. Provision of publicly funded MI-E devices shifted health care utilization and costs from the acute care to community sector, with evidence suggesting costs savings from reduced hospital days and fewer specialist visits.
Methods
Study Design, Setting, and Sample
We conducted a retrospective before and after clinical cohort study with subsequent linkage to health administrative databases held at ICES (formerly the Institute for Clinical Evaluative Sciences). ICES is an independent, nonprofit research institute whose legal status under Ontario’s health information privacy law allows it to collect and analyze health care data for health system evaluation and improvement. We prospectively recruited individuals who were approved for an MI-E device and used their health administrative data to determine health care utilization and associated costs 12 months before and 12 months after receipt as well as their 12-month survival trajectory. The study was conducted in the Canadian province of Ontario with participant recruitment coordinated through the Ventilator Equipment Pool, the organization responsible for providing publicly funded MI-E devices.
Study eligibility criteria comprised (1) meeting criteria for a publicly funded MI-E device via the Ministry of Health’s Assistive Devices Program with an application made and awaiting device delivery, (2) valid Ontario Health Insurance Plan (OHIP) number, (3) ability to speak and read English, and (4) consent to participate. We excluded individuals who had received an MI-E device for home use in the preceding 12 months. Individuals newly commenced on MI-E were identified consecutively and recruited over the telephone within 4 weeks of receiving their MI-E device by research staff at the Ventilator Equipment Pool.
Data Sources
We collected data from participants on demographic characteristics, primary medical diagnosis, use of assisted ventilation, number of medical devices used in the home, and ambulatory status over the telephone using a case report form following informed consent procedures. We then linked our cohort using unique encoded identifiers to Canadian Institute of Health Information and Ministry of Health administrative databases to identify (1) hospitalizations, ICU admissions, and in-hospital death from the Discharge Abstract Database; (2) ED presentations and same-day surgery from the National Ambulatory Care Reporting System; (3) physician billings including procedures from the OHIP physician claims database8; (4) prescription costs incurred in the community for individuals eligible for the Ontario Drug Benefit program; (5) in-patient rehabilitation from the National Rehabilitation Reporting System; (6) facility-based continuing (residential) and long-term care services from the Continuing Care Reporting System and the Client Profile Database; (7) in-patient mental health stays from the Ontario Mental Health Reporting System; (8) home care services from the Home Care Database; and (9) death outside of hospital from the Registered Persons Database. These data sets were linked using unique encoded identifiers and analyzed at ICES. Following well-established methods, both neighborhood income and urban/rural place of residence were ascertained using postal codes and linking to Statistics Canada census data.9
Study Outcomes
Our primary outcome was the number of presentations to the ED (all cause, respiratory related, and device related) in the 12 months before and 12 months after commencing MI-E therapy in the home. Secondary outcomes included all publicly funded health care utilization both in-hospital and in the community, health care costs, and 12-month survival trajectory. For those participants eligible for the Ontario Drug Benefit program, we also examined changes in drug prescriptions in the 12 months before and 12 months after commencing MI-E therapy in the home. Health care utilization included number of admissions to hospital (overall, respiratory related, and device related) and ICU; number of days admitted to hospital and to the ICU; same-day surgery visits; specialist physician visits (overall and respiratory related); family practice visits, home care visits (case management, nursing, homemaking and personal support, physiotherapy, occupational therapy, miscellaneous), and admission to a rehabilitation or long-term care unit.
We calculated health care utilization costs in CAD 2019 using established patient-level costing methodology10 then converted to USD (CAD $1 = USD 0.78). We calculated overall, total hospital, and total community costs as well as individual costs related to ED and ambulatory clinic visits; acute care hospital admissions; same-day surgery; physician and nonphysician billings; laboratory billings; out-of-hospital publicly funded prescription costs; home care; complex and continuing care; and residential long-term care.11
Ethical Considerations
The use of data held at ICES was authorized under section 45 of Ontario’s Personal Health Information Protection Act according to privacy regulations of ICES. Recruitment of participants to form the study cohort was approved by the University of Toronto (# 32677) and Queen’s University Health Sciences and Affiliated Teaching Hospitals (# 6017749) Research Ethics Boards.
Statistical Analysis
We present subject characteristics, health care utilization, and costs as medians and interquartile ranges (IQR) due to skewed data distribution. Unadjusted comparisons of pre/post differences were quantified using standardized differences12 and significance tests (Wilcoxon signed-rank tests for changes in count variables and McNemar test for changes in binary variables). Predictors of post-device health care utilization were identified using negative binomial regression models as overdispersion was present. A priori we considered the following variables potentially associated with health care utilization and costs: (1) demographic characteristics (age, sex, income quartile, rural residence), (2) clinical characteristics (need for ventilation, primary diagnosis, ambulatory status, number of medical devices used in the home), and (3) health care use in the year preceding MI-E approval. Given the rapidly progressive nature of amyotrophic lateral sclerosis (ALS) relative to other conditions, we included this diagnosis as a binary variable in our models of health care utilization and costs. Due to the relatively small cohort size and number of potential variables, we used forward selection to identify final regression models.
Health care costs before and after MI-E approval were log transformed due to skewed data for modeling purposes. We created 2 ordinary least squares regression models; one considered costs of only those individuals who remained alive during the 12 month follow-up; the second included all participants but assumed if people had lived they continued to incur health care costs at the same rate prior to their death. Model fit was examined using a quantile-quantile plot of residuals. To examine time to death, we created a Kaplan-Meier curve and a Cox proportional-hazard model to explore predictors. Lack of proportionality was examined by adding interactions between predictors and log(time).
All analyses were conducted by an experienced analyst using SAS version 9.4 (SAS Institute, Cary, North Carolina). All analyses were 2-tailed, P value of ≤ .05 considered significant.
Results
From June 2016 to February 2019, we recruited 108 participants. Of the 108 participants, 2 were excluded from ICES data linkage due to < 1 y of holding a valid OHIP number and, therefore, < 12 months of predevice health care data. Cohort demographic and clinical characteristics are shown in Table 1. Eight of the 106 participants were children age < 15 y. The most common indications for MI-E were ALS and muscular/myotonic dystrophies. Thirty-two percent of the cohort was not using mechanical ventilation (invasive or noninvasive) at the time of MI-E approval.
Demographic and Clinical Characteristics
Health Care Utilization
There was no difference in the rates of ED visits, overall or with a respiratory or device indication. There was also no difference in the number of hospital admissions before and after MI-E approval. However, participants required fewer d in hospital following MI-E approval (median IQR 0 [0–9] vs 0 [0–4], P = .03). Rates of physician specialist visits were also fewer following MI-E approval, both overall (median IQR 7 [4–11) vs 4 [2–7], P < .001) and to respiratory specialists (median IQR 2 [1–2] vs 1 [0–1], P = .002). Conversely, rates of home care nursing and homemaking/personal support visits increased following MI-E approval (Table 2). Use of health care in the year before MI-E approval was the most consistent predictor of health care encounters after MI-E approval. A diagnosis of ALS was not associated with respiratory (P = .70) or all-cause ED visits (P = .80), hospital admissions (P = .11), or d in hospital (P = .70). Being female was associated with fewer d in hospital, whereas increasing number of medical devices used in the home was associated with more d in hospital (Table 3).
Health Care Utilization 12 Months Before and After MI-E Initiation
Variables Associated With Patterns of Health Care Utilization Following MI-E Approval
For the 64 participants eligible for the Ontario Drug Benefit program, the proportion of individuals prescribed an inhaled medication decreased (22 [20.8%] vs 12 [11.3%], P = .008) as did the median (IQR) number of inhaled medications (0 [0–1] vs 0 [0–0], P = .01). We found no difference in the number of antibiotic/anti-infective medications prescribed. The median (IQR) number of cardiac drugs prescribed per individual also decreased (4 [0–9] vs 2 [0–10], P = .03). Conversely, a higher proportion of individuals was prescribed analgesics following MI-E approval (19 [17.9%] vs 29 [27.4%], P = .01), with individuals being prescribed a greater median (IQR) number of analgesics (0 [0–2] vs 0 [0–3], P = .02). The median (IQR) number of mood-enhancing drugs also increased following MI-E approval (2 [0–6] vs 4 [0–10], P = .003).
Health Care Costs
For 59.4% of the cohort, total costs was lower after MI-E device approval; 13.2% had no difference in costs, and 27.4% had higher costs. When examined more closely, total hospital costs (including in-patient stays, ED visits, and same-day surgeries) were not different before and following MI-E approval; however, other costs differed. Specifically median (IQR) physician billing ($2.474 [$1,349–$4,986] vs $2,090 [$1,224–$3.489], P = .02) and laboratory billing costs ($73 [$0–$128] vs $0 [$0–$62], P < .001) were lower following MI-E approval. Conversely, home care costs were higher, resulting in no difference in the median (IQR) total costs ($19,686 [$7,421–$39,932] vs $20,519 [$8,380–$40,401], P = .72) (Table 4).
Health Care Costs in 12 Months Before and After MI-E Initiation
Modeling indicated health care costs prior to MI-E approval were the most important predictor of costs following MI-E approval for those without ALS. However, preapproval costs were not associated with costs following MI-E approval for subjects with ALS (See Table 5 for modeling of data considering only those participants alive at 12-month follow-up). Ambulatory status, age, and diagnosis were associated with higher costs following MI-E approval, with nonambulatory subjects with ALS having the highest costs and ambulatory and non-ALS subjects between the ages of 35–49 having the lowest costs. All diagnostic groups had lower costs than ALS following MI-E approval except children with spinal muscular atrophy (see related supplementary material at http://rc.rcjournal.com).
Predictors of Health Care Costs for the 83 Participants Alive at 12 Months
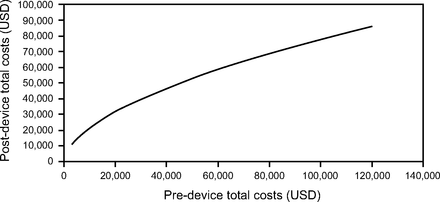
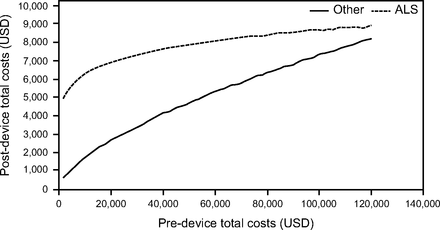
Costs following MI-E approval increased by 5.2% for each 10% increase in costs incurred beforehand. Figure 1 shows that in nonambulatory subjects with ALS age ≥ 80 (the highest-cost group) whose costs before MI-E were relatively low, costs following approval were much higher (eg, health care costs of $2,340 before and $14,040 after MI-E approval). However, as preapproval costs increased, the difference in costs between before and after narrowed (eg, health care costs of $93,600 before and $101,389 after MI-E), suggesting use of MI-E in the home decreased the cost differential, resulting in cost savings. If considering only ambulatory individuals in the lowest age category (35–49-y-olds), we found substantial cost savings following MI-E approval (Fig. 2).
Predicted costs for a nonambulatory participant with ALS (ie, reference case).
Predicted costs for an ambulatory participant in the lowest-cost age group (age 35–49 y).
Survival
Of the 106 participant cohort, 8 (7.5%) died within 6 months and 23 (21.7%) with 12 months. Risk of death was higher for older participants (hazard ratio [HR] 1.34 for each additional 10 y of age, 95% CI 1.10–1.65) and those using more medical devices in the home (HR 1.12 per additional device, 95% CI 1.02–1.22). Risk of death was lowest in those participants not using any form of assisted ventilation (HR 0.30, 95% CI 0.12–0.72 compared to noninvasive ventilation). Invasively ventilated subjects had a higher risk of death; however, this was not statistically significant due to small participant numbers (HR 3.71, 95% CI 0.71–19.45 compared to no assisted ventilation).
Discussion
In this 106-participants (adult and children) retrospective before and after cohort study with linkage to real-world health care utilization and cost data, we found individuals approved to receive an MI-E device for use in the home due to neuromuscular respiratory insufficiency spent fewer d in hospital and required fewer physician specialist (any specialist and respirologist) clinic visits following MI-E approval. We identified no difference in terms of rates of ED presentation, but use of home care services was increased. We previously identified a similar pattern of reduced hospitalization and increased use of home care services following initiation of home ventilation.13 Prescriptions for inhaled medications decreased after MI-E approval, but prescriptions of antibiotics and other anti-infective agents remained similar. Cost savings related to physician and laboratory billing costs were negated by increased costs of home care and community services. However, modeling of health care costs before and after MI-E approval suggested a decrease in the cost differential indicating cost savings with MI-E.
To our knowledge, this is the first study to quantify the effects of MI-E used by individuals with neuromuscular disorders in the home on public health care utilization, public health care costs, and survival trajectory. Very limited contemporary data exist regarding long-term clinical outcomes and resource use in other jurisdictions to enable comparison. In adults, despite multiple studies demonstrating improvements in cough peak flow and other lung function parameters,1,14,15 and clinical guidelines recommending use of MI-E in the home,16,17 we identified only one previous study reporting long-term clinical outcomes. In a prospective study of 39 adults with ALS, Vitacca and colleagues18 evaluated a telephone-accessed consultation program with MI-E and manually assisted coughing and oximetry feedback. Of the 24 participants that received MI-E, 20 (83%) were hospitalized in the follow-up period (mean SD 7.5 [5.8] months). However, the authors estimated 64% of potential hospitalizations was avoided (defined as relief of dyspnea and return of 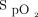 to baseline of 95%) with the program. We did not detect differences in patterns of health care utilization before and after MI-E compared to other diagnoses even though this could be hypothesized due to the more rapidly progressive nature of ALS and may actually reflect some benefit of MI-E.
to baseline of 95%) with the program. We did not detect differences in patterns of health care utilization before and after MI-E compared to other diagnoses even though this could be hypothesized due to the more rapidly progressive nature of ALS and may actually reflect some benefit of MI-E.
In children, studies also demonstrated improvements in lung function parameters,19,20 and clinical guidelines recommend use of MI-E in the home.21,22 Two small retrospective studies reported clinical outcomes. One study of 37 children reported respiratory-related hospital admissions and number of admission d were reduced after the introduction of MI-E.23 Similarly, an earlier retrospective cohort of 10 children reported a reduction in hospital days at 12 months following commencement of home MI-E compared with the same period before but no difference in hospitalization rates.24
In our study cohort, total real-world public health care utilization costs were approximately US $19,500 in the year before and the year after MI-E device approval. This estimate is relatively similar to our previously estimated annual public health care utilization costs of living at home on mechanical ventilation (US $22,558).25 Of note, public health care costs underestimate the total costs of health care as they fail to consider costs associated with family caregiving, private out-of-pocket, and third-party insurance costs. Although total overall costs before and after MI-E device approval were not different, our modeling indicated that costs incurred prior to MI-E approval were the most important predictor of costs for individuals without ALS. We also identified cost savings as public health care costs were proportionately lower following MI-E compared to costs incurred before MI-E. Furthermore, shifting of health care utilization and associated costs from acute care to the community sector, with fewer hospital days and enhanced community support, would be viewed positively from a patient and family perspective.
Limitations
This study has limitations related to its retrospective nature. First, this study is subject to the limitations inherent to administrative database studies in terms of data availability and quality. Use of a prospectively identified cohort enabled us to accurately identify primary medical diagnosis (as opposed to relying on ICD-10 codes), type of assisted ventilation, ambulatory status, and number of medical devices used in the home. Second, use of a before and after design without a control group who did not receive MI-E limits our ability to comments on causal effects of MI-E and ability to account for secular trends. Given that MI-E devices are now publicly funded and prescribed for all individuals meeting criteria for approval within this jurisdiction, there are substantial challenges to conducting a randomized controlled trial. Third, we were unable to account for adherence to prescribed MI-E therapy in our modeling. Finally, our data may not be generalizable to jurisdictions without publicly funded health care systems for acute and community health care provision where there will be greater reliance on private health insurance and different cost models. However, in countries with similar models of publicly funded health care services, the patterns of cost savings are likely to be replicable.
Conclusions
Provision of a MI-E device for management of neuromuscular-related respiratory complications in the home did not influence rates of ED visits or hospital admissions. However, it did alter health care utilization patterns, resulting in decreased hospital days and physician specialist visits but increased use of community and home care services. Cost savings related to physician billings were negated by increased costs of home care and community services; however, evidence supports public health care costs savings following provision of an MI-E device. Risk of death was highest in individuals requiring multiple forms of medical technology.
Footnotes
- Correspondence: Louise Rose PhD, Florence Nightingale Faculty of Nursing, Midwifery & Palliative Care, King’s College London, 7 Waterloo Rd, London, UK, SE1 8WA. E-mail: louise.rose{at}kcl.ac.uk
The authors disclose a relationship with IQVIA Canada.
This study was performed at the Ventilator Equipment Pool, Kingston, Canada and ICES, Toronto, Canada.
This study was funded by a grant from the International Ventilator Users Network. This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and the Ministry of Long-Term Care; and by the Canadian Institute for Health Information.
Supplementary material related to this paper is available at http://rc.rcjournal.com.
- Copyright © 2022 by Daedalus Enterprises