Abstract
Because some disease processes produce radiographic abnormalities that occur in characteristic distributions in the chest, classifying the position and appearance of these suggestive features and the underlying diseases provides a tool by which diagnostic accuracy might be improved. The goal of this review is to offer to the chest clinician a taxonomy of these disease entities that can produce characteristic chest radiographic distributions. These radiographic distributions often reflect anatomic or physiologic conditions that drive the radiographic appearance; for example, foramen of Morgagni diaphragmatic hernias most commonly present in the right ventral chest, consistent with the anatomic location of the diaphragmatic foramen. This taxonomy includes 3 distributional categories: (1) upper versus lower lung zone-predominant processes, (2) central versus peripheral processes, and (3) processes with distinctive focal locations, eg, “photonegative appearance” as in chronic eosinophilic pneumonia. It is hoped that this taxonomy aids the chest clinician in generating and streamlining a differential diagnosis and in ascertaining the specific cause of diseases with radiographic abnormalities.
Introduction
The distribution of radiographic abnormalities strongly conditions the differential diagnosis in chest medicine.1 The distribution of pathologic processes in the lung is affected by ventilation and perfusion patterns, where both ventilation and perfusion demonstrate an apical-basal gradient. More specifically, perfusion of the apices amounts to only 5% of perfusion of the lung bases and ventilation of the apices only 30% of basal ventilation.1 The result is that the ventilation/perfusion (V̇/Q̇) ratio is highest at the lung apex (2.1) than at the lung base (0.3), causing enhanced oxygenation at the lung apex and presumably predisposing to some apical diseases like reactivation Mycobacterium tuberculosis.2
Lymphatic clearance of intra-alveolar material (eg, inhaled exogenous particles) also impacts the radiographic distribution of disease and is much more vigorous in the lung bases than the apices. As a result, organic antigen-related diseases like hypersensitivity pneumonitis may be upper lung zone-predominant, where lymphatic clearance is less robust. Finally, mechanical factors may affect radiographic distribution. When the integrity of lung parenchyma is compromised as in centriacinar emphysema, it makes sense that the lung apices, which are under greater mechanical stretch and strain, are predominantly affected. Similarly, gravity may predispose to unilateral pulmonary edema in individuals spending prolonged periods in the lateral decubitus position.
In addition to apicobasilar patterns, distribution in the transaxial plane (ie, centrally vs peripherally in the chest) also guides the differential diagnosis. Specifically, some processes predominantly affect the central (or peribronchovascular) areas of the chest, whereas others preferentially involve the peripheral (or subpleural) regions. Finally, some diseases cause characteristically focal infiltrates, such as segmental or lobar edema seen in the right upper lobe due to a mitral regurgitation jet.3
To enhance the chest clinician’s interpretation of both plain chest radiographs and/or chest computed tomography (CT) images and diagnostic recognition of entities with characteristically suggestive radiographic distributions, the current paper offers a pictorial catalog of diseases that feature distinctive apical-basal, transaxial, or distinctively focal radiographic abnormalities. Whereas it goes without saying that no radiographic pattern is pathognomonic of any entity and that exceptions to any classic pattern are well documented to occur, it is hoped that this catalog of classic patterns will enhance the chest clinician’s diagnostic acumen and facilitate diagnosis.
Processes With Distinctive Vertical (Upper vs Lower Lung Zone) Distributions
Some entities characteristically affect the upper lung zones and others the lower lung zones (Table 1).
A Catalog of Diseases Commonly Characterized by a Distinctive Radiographic Distribution
Upper Lung Zone-Predominant Processes
Diseases that are characteristically upper lung zone-predominant include selected infections and inflammatory conditions, inhalational diseases, centriacinar emphysema, and Pancoast tumors.
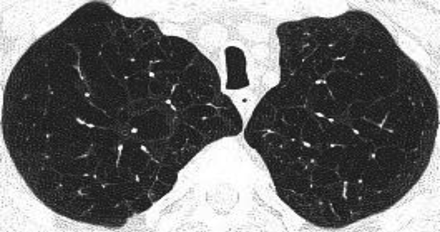
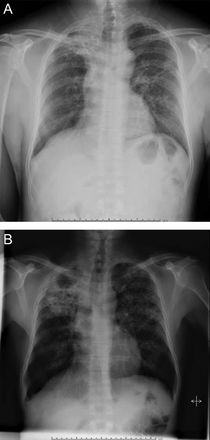
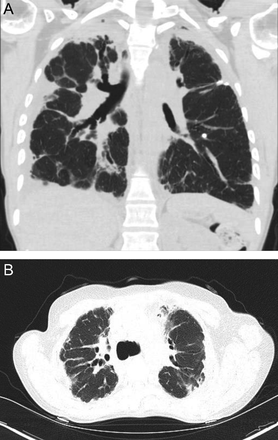
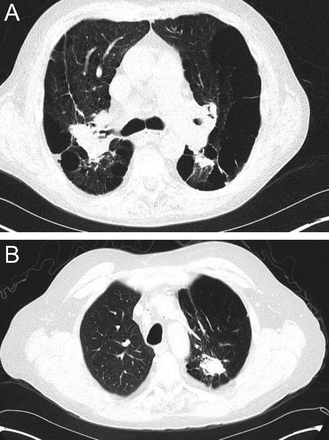
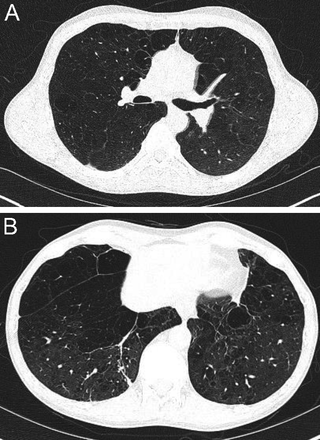
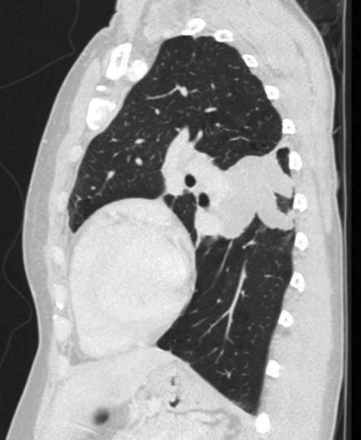
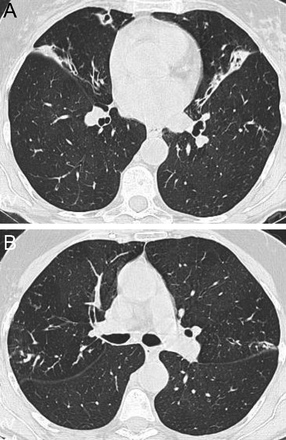
Centriacinar emphysema is the anatomic type of emphysema that is usually associated with cigarette smoking.4 In contrast to the distribution of emphysema in alpha-1 antitrypsin deficiency, which is characteristically lower lung zone-predominant, emphysema associated with cigarette smoking (in individuals with normal alpha-1 antitrypsin levels) disproportionately affects the upper lung zones. In a series by Stavngaard et al5 centriacinar emphysema was upper lung zone-predominant in 84% of cases (Fig. 1). Reactivation M. tuberculosis is the classic process that characteristically involves the upper lung, most commonly involving the apical segments of the upper lobes or the superior segments of the lower lobes (Fig. 2). In one series, Woodring et al6 described the involvement of apical and posterior segments of the upper lobe in 91% of the 56 cases with post-primary tuberculosis. This predilection for the upper lung zones has been ascribed to the favorable V̇/Q̇ ratios in the upper lung zones. Fungal infections such as Histoplasma can mimic this apical distribution, as can chronic melioidosis. In a series of 183 chest radiographs of individuals with melioidosis from Thailand, Dhiensiri et al7 reported that among the 31 individuals with chronic melioidosis 13 had single-lobe involvement that involved the upper lobe in 92%.
Chest computed tomography (axial cut from the upper lung) showing centriacinar emphysema. Characteristic features include diffuse “holes” with no walls, surrounding small vessels, and minimal preserved peripheral normal lung. In individuals with smoking-related emphysema who have normal alpha-1 antitrypsin levels (ie, do not have alpha-1 antitrypsin deficiency), the emphysematous changes are disproportionately distributed in the upper lung zones.
Chest radiographs showing reactivation post primary Mycobacterium tuberculosis with right upper lobe consolidation progressing to cavity formation (A). Note worsening reticular and tiny nodular opacities in the left upper lobe suggestive of active bronchogenic spread.
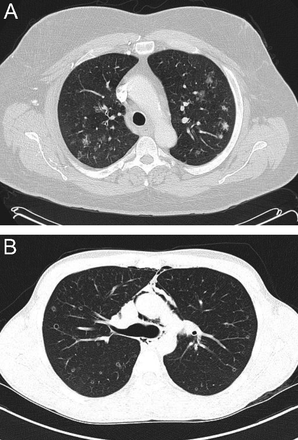
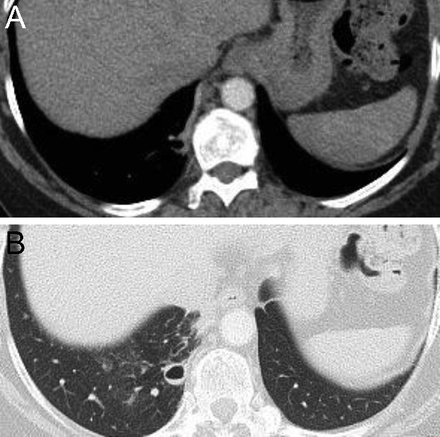
Finally, in addition to reactivation M. tuberculosis and chronic melioidosis, Pneumocystis jirovecii that occurs in patients receiving aerosolized pentamidine as prophylaxis characteristically manifests as upper lung zone infiltrates, presumably reflecting the ventilation patterns that cause less of the pentamidine to be delivered to the upper lobes8 (Fig. 3).
Chest computed tomography (apical cut) in a patient with AIDS and severe hypoxemia due to Pneumocystis jirovecii pneumonia that developed despite prophylactic aerosolized pentamidine.
Among inflammatory diseases, sarcoidosis, pleuroparenchymal fibroelastosis, the apical fibrobullous pattern in rheumatoid arthritis, and ankylosing spondylitis are upper lung zone-predominant. The apical changes associated with ankylosing spondylitis and rheumatoid arthritis can be cavitary and mimic infection, though it is recognized that rheumatoid arthritis can have many other chest manifestations (eg, a pattern of usual interstitial pneumonia [UIP], which is characteristically lower lung zone-predominant, pleural effusions, cavitary nodules [called Caplan syndrome]). In a series of 2,080 subjects with ankylosing spondylitis, Rosenow et al9 identified 28 (1.3%, all men) with pleuropulmonary manifestations; 26 of these had apical fibrobullous change, usually bilateral. In some instances, the bullous changes were comprised of cysts in which aspergillomas subsequently developed.
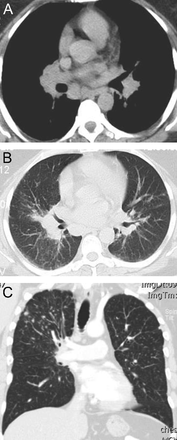
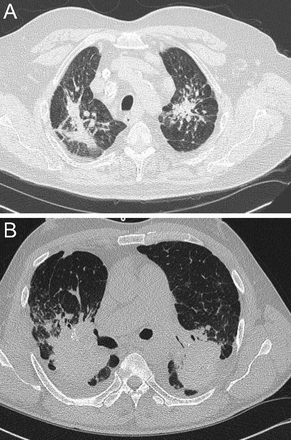
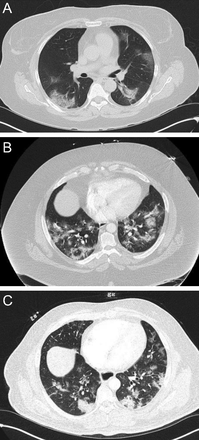
Although sarcoidosis may have protean radiographic chest manifestations, sarcoidosis also classically affects the upper lung zones (Fig. 4). For example, upper lobe fibrosis composes one of the criteria justifying “at least probable” lung involvement with sarcoid in the World Association of Sarcoidosis and Other Granulomatous Disorders criteria to define organ involvement.10
Chest computed tomography cuts. A and B show stage 2 sarcoidosis: bilateral hilar/subcarinal lymphadenopathy and peribronchovascular nodular densities in both lungs. Stage 3 sarcoidosis on coronal (right) reformatted images demonstrate multiple 2–4 mm nodules along the bronchial and vascular walls of both upper lobes (C).
Pleuroparenchymal fibroelastosis is a fibrotic process that involves the pleura and the subpleural lung parenchyma, characteristically beginning and progressing from the lung apices.11 As evidence of the characteristic upper lung zone-predominant nature of pleuroparenchymal fibroelastosis, diagnostic criteria for include upper lung zone pleural thickening; apical subpleural parenchymal fibrosis; and limited, if any, lower lung zone involvement12 (Fig. 5).
Pleuroparenchymal fibroelastosis. Coronal (A) and (B) axial chest computed tomography show biapical soft-tissue thickening, subpleural fibrosis, and traction bronchiectasis.
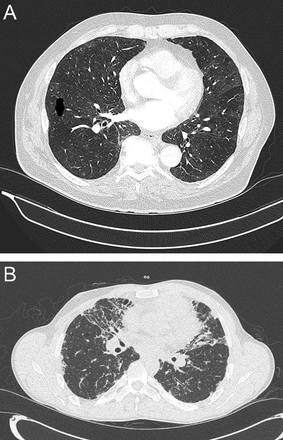
Finally, pulmonary Langerhans cell histiocytosis characteristically shows symmetric, diffuse reticulonodularity and involves the upper lung zones while sparing the costophrenic angles. In a French series of 50 subjects with pulmonary Langerhans cell histiocytosis, Lacronique et al13 reported that the costophrenic angles were spared in 84% of the chest radiographs (Fig. 6).
Pulmonary Langerhans cell histiocytosis in 2 different young adults, both active smokers. A: (left) Axial computed tomography (CT) cut in a patient presenting with cough. Note multiple irregular centrilobular nodules in both upper lobes and smooth bronchial wall thickening. B: (right) Axial chest CT cut at level of the main carina showing a pneumomediastinum due to ruptured cyst(s) in an otherwise asymptomatic patient. Note multiple thin-walled small cysts in both upper lobes and mid lung zones.
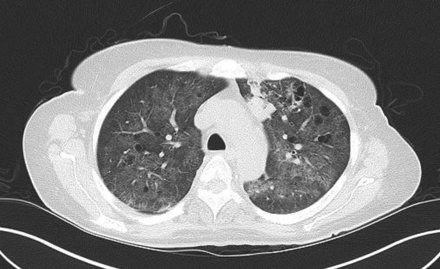
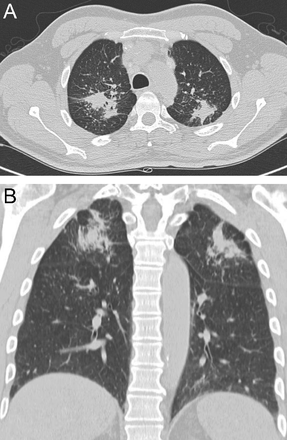
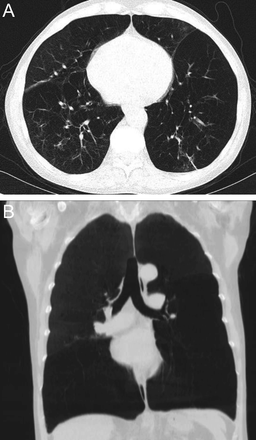
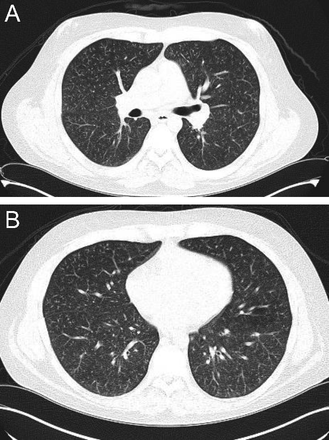
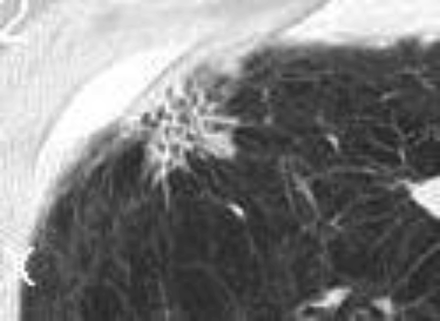
Hypersensitivity pneumonitis is an allergic granulomatous response to an antigen, often as a result of inhalation of organic dust and subsequent inflammation. Centrilobular nodules along with a mosaic pattern of perfusion with ground glass attenuation are characteristic features (Fig. 7). Adler et al14 reported a series of 16 patients with chronic hypersensitivity pneumonitis and found middle lung zone predominance in 44% of the patients, whereas in 38% of the patients there were no specific distribution.
Axial cuts from a chest computed tomography in a patient with hypersensitivity pneumonitis demonstrating small bilateral centriacinar nodules in an upper lobe distribution.
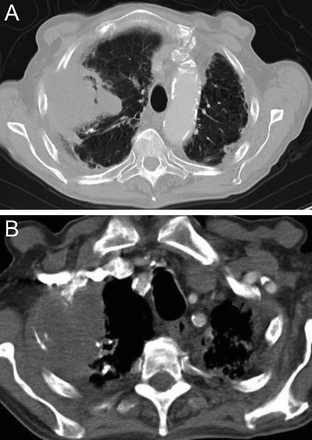
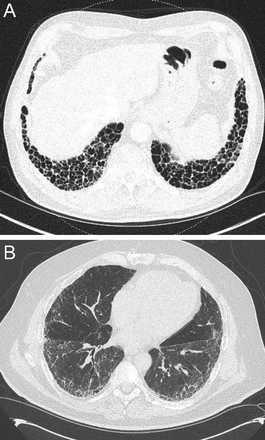
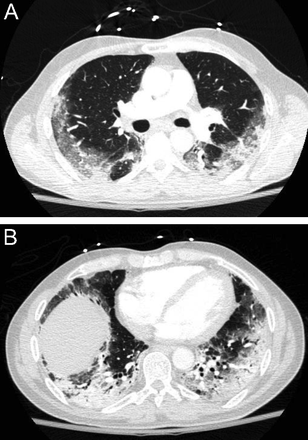
Among the occupational lung diseases (or pneumoconioses), silicosis and coal workers’ pneumoconiosis (CWP) show an upper lung zone predominance.15 Chronic silicosis is characterized by innumerable small rounded nodular opacities in the upper lung zones (Fig. 8). Over time, these smaller nodular opacities coalesce and form large upper/middle lobe opacities called conglomerate silicosis or progressive massive fibrosis (PMF) (Fig. 9). CWP is often indistinguishable from silicosis on imaging (Fig. 10). In a series of images from 138 coal miners with 30 y of exposure at work who had PMF, Wade et al16 reported that the average time to progression from a normal chest radiograph to PMF was 12 y.
Axial (A) and coronal reformatted chest computed tomography images in a patient with silicosis demonstrate large conglomerate masses (A and B) with multiple tiny dense perilymphatic satellite nodules in the posterior upper lobes (A).
Axial cuts of a chest computed tomography in a patient with progressive massive fibrosis (PMF) show large perihilar lung masses consistent with PMF with development of pericicatricial emphysematous changes (especially evident on the left side) between the masses and the adjacent chest wall pleura.
Coal workers’ pneumoconiosis (CWP). Noncontrast chest computed tomography images in lung window settings in 2 different patients with CWP showing many bilateral tiny centrilobular nodules with upper-lobe predominance. A: 77 y old male coal miner with simple CWP (left). B: Complicated CWP with large, irregular fibrotic masses in both upper lobes in a 62 y old male consistent with progressive massive fibrosis (right).
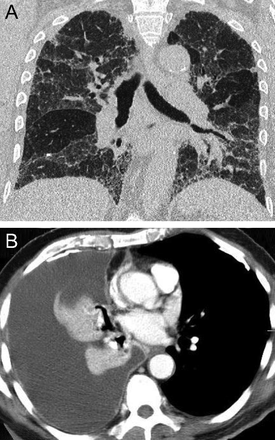
The so-called Pancoast or superior sulcus tumor is defined by its upper lobe location, characteristically adjacent to the subclavian vessels on either side of the vertebral column and above the anterior portion of the first rib17 (Fig. 11). The upper-lobe proximity to the ribs, shoulder, and stellate ganglion accounts for the common presenting signs and symptoms of Pancoast tumors, which include shoulder pain and arm pain in a dermatomal distribution of C8, T1, and T2; upper extremity muscle weakness; and the Horner syndrome (ptosis, miosis, anhidrosis).
Axial unenhanced chest computed tomography at the level of the lung apices showing a Pancoast tumor. (A, left) Soft tissue and (B, right) lung window images reveal a large, peripheral, pleural-based mass with heterogenous attenuation (solid and necrotic/cystic components) in the right upper lobe. The right first and second ribs are partly destroyed.
Lower Lung Zone-Predominant Processes
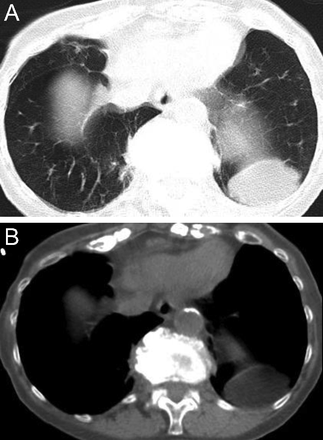
Unlike centriacinar emphysema that predominantly affects the upper lung zones, emphysema associated with severe deficiency of alpha-1 antitrypsin predominantly affects the lower lung zones18 (Fig. 12). In a series of 102 chest CT scans of PI*ZZ alpha-1 antitrypsin–deficient individuals, Parr et al19 reported that 64% had predominantly lower lobe emphysematous involvement.
Axial (A) and coronal (B) chest computed tomography images in a patient with alpha-1 antitrypsin–associated emphysema showing panlobular emphysema. There is complete replacement of normal lung architecture in both lower lobes with lucent lung parenchyma. Coronal reformatted minimum-intensity projection image (B, right) clearly shows lower lobe predominance of panlobular emphysema with subtle emphysematous changes detected bilaterally in the upper lobes.
Other entities like the emphysema accompanying hypocomplementemic urticarial vasculitis (Fig. 13) or that associated with intravenous talc injection (eg, accompanying intravenous heroin or methylphenidate use) can mimic this lower lung zone-predominant pattern of emphysema20 (Fig. 14). Other characteristically lower lung zone processes include UIP, non-specific interstitial pneumonia (NSIP), asbestosis, alveolar microlithiasis, aspiration pneumonia, and hematogenous metastases from tumors originating outside the chest.
Axial chest computed tomography images in a patient with hypocomplementemic urticarial vasculitis showing lower lobe-predominant emphysema. The differential diagnosis includes alpha-1 antitrypsin deficiency and intravenous methylphenidate (Ritalin) abuse.
High-resolution chest computed tomography images through the upper (A, left) and middle (B, right) lung zones in a 32 y old intravenous drug abuser showing diffuse bilateral small nodules of injection talc granulomatosis.
UIP is characterized by bilateral, basal subpleural reticular opacities, honeycombing, and traction bronchiectasis (Fig. 15). In a series of 22 subjects with UIP, Elliot et al21 noted lower lobe predominance in 85%.
Axial chest computed tomography cuts in a patient with usual interstitial pneumonitis with classic, multilayered, honeycomb cysts (A, left) in the subpleural and basilar portions of both lungs with traction bronchiectasis.
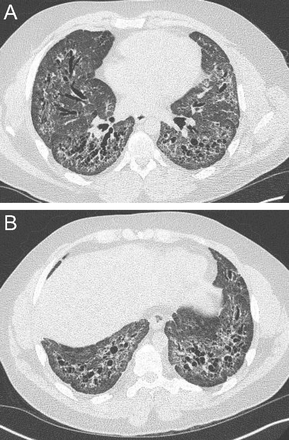
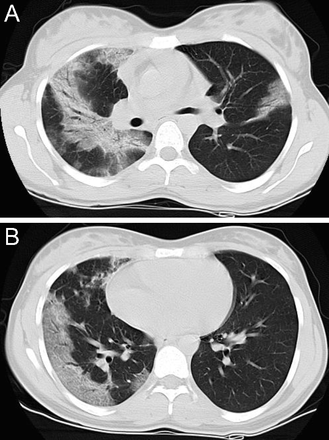
NSIP, characterized by bilateral, subpleural distribution of ground glass attenuation, linear irregular opacities, consolidation, and bronchial dilation, has a lower lung zone predominance. In a series of 50 subjects with biopsy-proven NSIP (Fig. 16), Hartman et al21 reported ground glass attenuation in 76% of the cases, with predominance in the lower lung zone in 59% of the cases; also, irregular linear opacities were noted in 22% of the subjects, with lower lung zone involvement in 87%. Additionally, in the aforementioned series by Elliot et al,22 lower zone predominance was observed in 90% of 25 subjects with NSIP. Finally, in a case series of 7 subjects with NSIP by Park et al,23 6 had bilateral patchy ground glass opacification with a lower or middle lung zone predominance, and Nishiyama et al24 reported a series of 15 subjects with a cumulative 80% prevalence of lower or mid-lower lung zone abnormalities; 26.7% demonstrated lower, and 53.3% had mid-lower lung zone involvement.
Axial chest computed tomography cuts in a patient with non-specific interstitial pneumonitis (NSIP): lower lobe–predominant peribronchial reticular opacities, ground glass opacities, and traction bronchiectasis in fibrotic NSIP. Note subpleural sparing is evident in NSIP.
Turning to idiopathic and other entities with lower lobe predominance, alveolar microlithiasis is a rare disorder characterized by bilateral, sand-like micronodular calcified densities (microliths). In a series of 5 subjects, Cluzel et al25 noted that these micronodular densities were predominantly in the lower two thirds of the lungs on CT chest, presumably related to the larger volumes of the lower lobes. Similarly, early asbestosis manifests on the chest radiograph as bilateral irregular parenchymal opacities in the lower lobes. On high-resolution CT chest, asbestosis causes subpleural linear opacities, basilar lung fibrosis, and honeycomb changes (Fig. 17A). Pleural plaques (Fig. 17B) indicate asbestos exposure.26
A: Chest computed tomography axial cut (lower lung zone) showing asbestosis. This image demonstrates diffuse interstitial fibrosis, usually lower lung zone–predominant, which is indicative of asbestosis. Advanced asbestosis is characterized by peripheral and posterior lung honeycomb-like changes, coarse reticulations, traction bronchiectasis, parenchymal bands, etc, which mimic idiopathic pulmonary fibrosis (usual interstitial pneumonitis pattern). B: Chest computed tomography axial cut in a patient with a pleural plaque and asbestos-related effusion. The calcified pleural plaque indicates asbestos exposure and can be present in isolation without evidence of asbestos-related parenchymal disease or other pleural disease. The full spectrum of asbestos-related pleural disease also includes benign pleural effusion, diffuse pleural thickening, rounded atelectasis, and mesothelioma.
Lastly, aspiration pneumonitis is a common entity that affects hospitalized patients. If an aspiration event occurs when the patient is recumbent, aspiration pneumonia is likely seen in the posterior segments of the upper lobe and superior segments of the lower lobes27 (Fig. 18). Landay et al28 reviewed the images of 60 subjects with aspiration pneumonia and reported the early appearance of infiltrates in 54. Of the 48 subjects with infiltrates on the initial chest radiograph, 31 were in the lower lung zones and 7 were in the mid lung zones, accounting for a cumulative 79% prevalence of basilar occurrence.
Axial chest computed tomography (CT) cuts in an elderly patient with stroke and preferred left-decubitus sleeping posture showing aspiration pneumonia in the left lower lobe (left, A). Initial CT chest shows left lower lobe pneumonia (left A), and subsequent CT (right, B) shows complete resolution of the left lower lobe pneumonia.
As commonly seen in patients with recurrent foreign matter aspiration, aspiration bronchiolitis is characterized by tree-in-bud opacities in the dependent-lung parenchyma, fluid in the major airways, airway wall thickening, and/or dependent-lung bronchiectasis (Fig. 19). Another example is chronic organizing diffuse alveolar damage, characterized by peripheral ground glass opacities and basilar predominant consolidation and traction bronchiectasis (Fig. 20).
Coronal chest computed tomography images (left, A) and axial (right, B) in a patient with aspiration bronchiolitis show many tree-in-bud opacities bilaterally in the lower lobes with bronchial wall thickening and mild right lower lobe bronchiectasis (right, B).
Axial chest computed tomography (CT) cuts in a patient with diffuse alveolar damage. Chest CT coronal reformatted images show peripheral ground glass opacities with basilar-predominant consolidations and traction bronchiectasis in chronic organizing diffuse alveolar damage.
Processes With Distinctive Transaxial (Central vs Peripheral) Distributions
In addition to distinctive distributions in a craniocaudal plane, some chest processes have distinctive distributions in the transaxial plane. Processes that characteristically affect the peripheral rather than the central lung fields (Table 1) include chronic eosinophilic pneumonia, organizing pneumonia, septic emboli, rounded atelectasis, so-called Hampton hump (which is a radiographic manifestation of pulmonary infarction characteristically complicating pulmonary embolism), and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS CoV-2).
CT manifestations of SARS CoV-2 pneumonia are protean, with vascular engorgement, ground glass opacities, subpleural bands, and interlobular septal thickening the most commonly described. Subpleural bands and a peripheral distribution of infiltrates are characteristic29 (Fig. 21).
Chest computed tomography axial cuts in a patient with COVID-19 pneumonia. Note the multilobar, peripheral, or subpleural ground glass opacities in both lungs with mid and lower zone predominance. Some of the opacities are vaguely ovoid in shape.
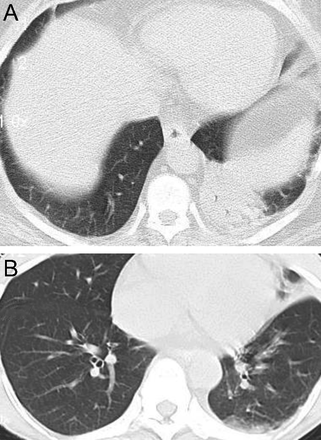
Chronic eosinophilic pneumonia is classically described as having a photonegative distribution, meaning that the infiltrates are characteristically peripheral with central sparing30 (Fig. 22). In a French series analyzing plain films of 62 subjects with chronic eosinophilic pneumonia, Marchand et al31 reported that all had peripheral alveolar infiltrates; changes were bilateral in 76%. Upper lung zone involvement was observed in 47% and lower lung zone changes in only 11%.
Axial chest computed tomography cuts in an 18 y old with a 3–pack-year smoking history, dry cough, and chronic eosinophilic pneumonia. There is nonsegmental peripheral and peribronchial air space opacities in both upper lobes (“photographic negative of pulmonary edema”).
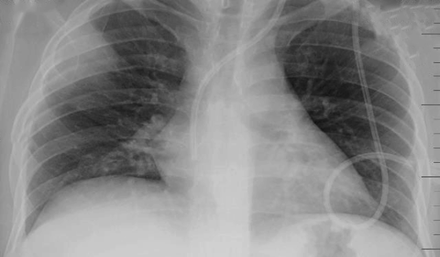
Hampton hump is a peripheral wedge-shaped density with a rounded convex apex that is directed toward the hilum (Fig. 23); Hampton hump results from pulmonary infarction, as may occur in pulmonary embolism. In an analysis of chest radiographs in 383 subjects with angiographically confirmed pulmonary embolism, Worsley et al32 observed a Hampton hump in 22%; other associated findings—the Westermark sign (focal hyperlucency due to oligemia) and Fleischner sign (prominence of the central pulmonary artery)—were found in 14% and 20% of plain chest radiographs, respectively. None of the findings had powerful predictive power for pulmonary embolism; the positive and negative predictive values for Hampton hump were 30% and 77%, respectively, and for Westermark sign 38% and 76%, respectively. Similarly, although septic emboli can have several non-specific presentations, they often manifest in the periphery of the lung as a nodule or nodules with a feeding vessel that can sometimes cavitate. Huang et al33 reported the CT findings in 15 subjects with septic emboli and found a peripheral nodule with a feeding vessel in 10 (67%).
Plain chest radiograph shows a Hampton hump in a patient with pulmonary embolism. There is a peripheral wedge-shaped density with a rounded convex apex that is directed toward the hilum on the right.
Entities that predominantly affect the central portions of the lung parenchyma include classic cardiogenic pulmonary alveolar edema (Fig. 24) and pulmonary alveolar proteinosis (Fig. 25), both of which manifest a “bat-wing pattern” of perihilar infiltrates. The bat-wing pattern in pulmonary edema was initially described by Werkenthein in 1939. Bronchogenic cancers like squamous cell carcinoma and small cell lung carcinoma are also characteristically central in the lung.
Plain chest radiograph in a patient with cardiogenic pulmonary edema showing a bat-wing infiltrate of pulmonary edema.
Axial chest computed tomography cuts in a patient with pulmonary alveolar proteinosis. The infiltrates show a pattern called “crazy paving” is a central, bat-wing distribution (left, A).
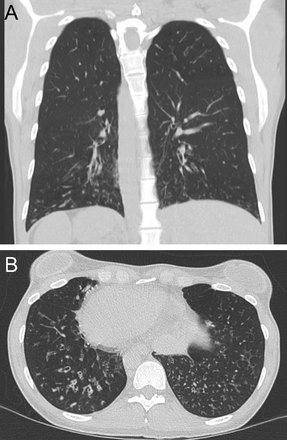
Allergic bronchopulmonary aspergillosis classically shows central bronchiectasis with evidence of mucus plugging on imaging. This was described by Mintzer et al34 as the “finger in glove” sign due to involvement of second-order bronchi (Fig. 26). Later, Agarwal et al35 evaluated 155 subjects with allergic bronchopulmonary aspergillosis and found bronchiectasis in 118 (76%) subjects, whereas the rest had normal CT findings.
Sagittal chest computed tomography image in a patient with allergic bronchopulmonary aspergillosis. The image shows dilated bronchi with mucoid impaction presenting as a branching tubular mass in the left lower lobe (“finger in glove” sign).
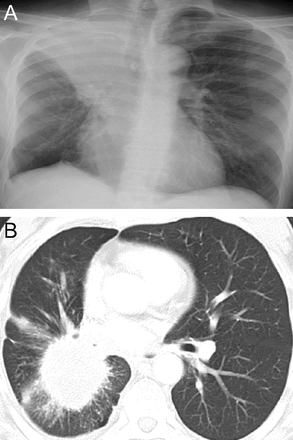
Small cell lung cancer characteristically presents as a central mass, is often seen endobronchially, and is often accompanied by mediastinal lymphadenopathy (Fig. 27). Watson and Berg36,37 first noted the predominantly central location of small cell carcinoma on chest radiographs and the early tendency for small cell carcinoma to metastasize. In contrast, adenocarcinoma of the lung is commonly seen as a peripheral lung mass38 (Fig. 28).
Plain chest radiograph (right, A) in a patient with squamous cell carcinoma of the lung. This chest radiograph shows a central right hilar tumor (squamous cell carcinoma lung) causing obstruction of the right upper lobe bronchus and right upper lobe atelectasis (“S sign of Golden”). The homogenous opacity in the right upper hemithorax has a sharp, somewhat S-shaped inferior margin (lateral aspect contributed by the superiorly retracted minor fissure and medial portion by the inferior margin of the mass itself). The axial chest computed tomography cut (right, B) shows a central small cell lung cancer.
Section from a chest computed tomography axial image in a patient with non–small cell lung cancer. The image shows a spiculated, peripheral right upper lobe subsolid nodule (non–small cell lung cancer) associated with emphysematous changes in a 50 y old male with a 60–pack-year smoking history.
Processes With Distinctive Focality in the Lung
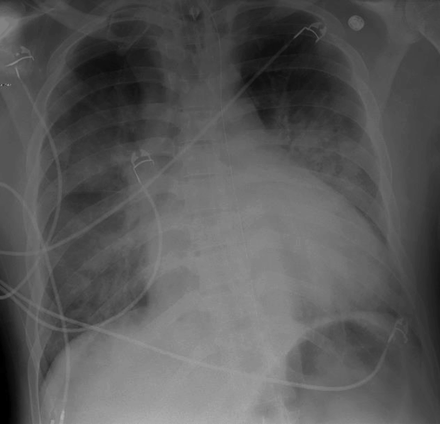
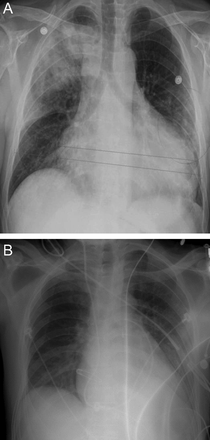
In addition to processes with a distinctive vertical or axial distribution, some entities are distinctive by virtue of their characteristic focality in the lung. As an example, focal pulmonary edema in the right upper lung zone may classically accompany severe mitral valve regurgitation39 (Fig. 29). In this instance, the regurgitant flow from the left ventricle is classically directed toward the right superior pulmonary vein, accounting for the unilateral pulmonary edema that causes the infiltrate. At the same time, focal infiltrates related to severe mitral regurgitation are not limited to the right upper lung zone. For example, in a series of 869 consecutive subjects with acute cardiogenic pulmonary edema, Attias et al40 reported unilateral pulmonary edema in 2.1% (18 subjects), all with severe mitral regurgitation. The distribution of unilateral pulmonary edema in these individuals was right sided in 89%, involved the upper lung zone in 37.5%, the upper and mid lung zones in 12.5%, the middle and lower lung zones in 25%, and the entire right lung in 25%. Notably, in 2 subjects (11%), the unilateral pulmonary edema complicating severe mitral regurgitation involved the left lung, with the entire left lung involved in one subject and the left upper lobe only in the other.
Chest radiographs showing flash pulmonary edema with very focal right upper lobe involvement (left, A) that resolved with treatment (right, B).
Other etiologies can cause unilateral pulmonary alveolar filling, many of which are not cardiogenic. Some entities are ipsilateral to the abnormality and others contralateral (Table 2). Calenoff et al41 catalogued 18 different causes of unilateral pulmonary edema, 9 of which were ipsilateral to the inciting cause (Table 2).
Causes of Unilateral Pulmonary Edema
Corrective surgeries for congenital heart disease (eg, Blalock-Taussig anastomosis, Waterstone procedure) redirect and increase blood flow, causing elevation of venous pressure either in the right chest (entity 1 in Table 2) or the left chest (entity 10). Occlusion of pulmonary veins due to a variety of causes (eg, veno-occlusive disease; structural blockage or compression of the pulmonary veins as by aortic dissection; following ablation therapy for atrial fibrillation, tumor, or granulomatous infection) can also increase venous return pressures and cause leakage of fluid into the alveoli.
Prolonged lateral decubitus positioning has been associated with ipsilateral pulmonary edema, as can unilateral aspiration. Rapid or large-volume aspiration of pleural fluid can cause ipsilateral alveolar fluid, with special caution when the lung is “trapped,” as by a thickened pleural peel. Similarly, aspiration of pleural air can produce ipsilateral alveolar flooding, presumably related to the sequelae of rapidly increasing pulmonary blood flow accompanying rapidly decreasing intrapleural pressures.
Regarding processes involving contralateral alveolar flooding, pulmonary artery hypoplasia or absence diverts blood contralaterally, with resultant alveolar flooding. When lung perfusion is compromised (as in congenital pulmonary artery hypoplasia accompanying alveolar hyperinflation [Swyer-James-MacLeod syndrome] or pulmonary thromboembolism), contralateral perfusion increases. When emphysema is focal, perfusion to the affected area of the lung may also decrease, thereby diverting blood flow elsewhere and predisposing to alveolar flooding in areas remote from the emphysema. Similarly, it has been proposed that lung that is compressed by encircling pleural disease experiences decreased perfusion such that cardiogenic edema will manifest contralaterally. Finally, ARDS has been described to cause contralateral pulmonary infiltrates in a patient with a prior thoracic sympathectomy, presumably due to dysregulation of venous post-capillary sympathetic-mediated tone.
Selected infections such as non-tuberculous mycobacteria characteristically cause bronchiectasis of the middle lobe and lingula in elderly women with impaired cough reflexes (named the “Lady Windermere syndrome”42,43 [Fig. 30]). In a series of 63 individuals with non-tuberculous mycobacterial infections, Kim et al44 reported that 95% were women of average age 60 y. They were taller and leaner than an age-matched population and more frequently had scoliosis (51%), pectus excavatum (11%), and mitral valve prolapse (9%). Infection with Mycobacterium avium intracellulare complex was most common, followed by Mycobacterium xenopi and Mycobacterium kansasii. Bronchiectasis characteristically involved the right middle lobe (90%) and lingula (73%), and the pattern of bronchiectasis was most frequently cylindrical (61%) and less commonly saccular (43%) or cystic (9%). Other radiographic features were nodules, tree-in-bud opacities, and cavities.
Axial cuts of a chest computed tomography in a patient with Mycobacterium avium intracellulare, commonly characterized by bronchiectasis in the middle lobe (A and B) and lingual (A).
A different entity, called the middle lobe syndrome, relates to atelectasis of the right middle lobe (and also occasionally the lingula, then called the lingula syndrome) either caused by extrinsic obstruction of the lobe (eg, by adjacent lymphadenopathy or mass) or without extrinsic obstruction (eg, speculated to be caused by the angularity of takeoff of the right middle lobe coupled with poor collateral ventilation). Pathologic findings in the middle lobe syndrome commonly include bronchiectasis and suppuration, with a low prevalence of neoplasm or tuberculous infection.45,46
Congenital lesions such as pulmonary sequestration typically involve the posteromedial portions of the lower lobes. Pulmonary sequestration is characterized by nonfunctioning lung tissue and is not connected to tracheobronchial tree in a normal fashion. Unlike normal lung tissue, a sequestration receives its blood supply from the systemic circulation (eg, a branch of the aorta or bronchial arteries). In a series of 24 subjects with proven bronchopulmonary sequestration reported by Ikezoe et al47 (16 of which were intralobar, ie, they lacked a discrete pleural covering), 88% (21 subjects) were located posterobasally, usually (71%) on the left side. Intralobar sequestration often presents later in life due to recurrent infection, whereas extralobar pulmonary sequestration (defined by having its own pleural surface) is commonly diagnosed antenatally or soon after birth. Collin et al48 reported that 98% of intralobar sequestrations occurred in the lower lobes. Characteristic features of pulmonary sequestration include it being a homogenous solid mass or a mixed solid and cystic mass in the lung base (left lower lobe more commonly than the right lower lobe). The presence of air lucencies may indicate infection, associated dysplastic lung, or a hybrid lesion with congenital pulmonary airway malformation; CT angiography demonstrates an aberrant systemic vascular supply (Fig. 31).
Contrast-enhanced chest computed tomography images in mediastinal (left, A) and lung (right, B) window settings in a patient with intralobar bronchopulmonary sequestration. There is a characteristic, well-circumscribed, cystic lesion in the posteromedial right lung base. Note the systemic feeding artery from the descending thoracic aorta (A).
Pleural pseudotumors refer to mass-like opacities caused by loculated pleural effusions along the fissures. They present as lens-shaped, biconcave opacities that can develop in any of the fissures but most commonly in the minor fissure. Higgins et al49 reported a series of 41 cases with loculated interlobar pleural effusion due to congestive heart failure, sometimes called “vanishing tumors” as they may disappear with diuresis; 78% of the effusions were located in the right transverse (minor) fissure.
Finally, openings or foramina in the diaphragm can present in characteristic locations in the chest. Foramen of Bochdalek hernia, named for the Czech anatomist Bochdalek who first described it in 1848, results from failure to close the pleuroperitoneal canal and characteristically occurs posteriorly on the left side50 (Fig. 32). In contrast, foramen of Morgagni hernias are characteristically right sided and anterior.51 Such congenital diaphragmatic hernias are estimated to occur in 1:2,200–1:2,500 live births and often do not become apparent until middle age. Comer et al52 reported a series of 50 subjects who underwent surgery for foramen of Morgagni hernia and noted that in 45 subjects the hernia was in the right parasternal region. More recently, Minneci et al53 reported a series of 12 subjects with foramen of Morgagni hernias who underwent surgical repair over a 15-y period and found that all hernias were right sided.
Axial chest computed tomography cuts in lung (left, A) and mediastinal (right, B) window settings in a patient with a foramen of Bochdalek hernia, which is located posteriorly on the left.
Summary
Distinctive radiographic patterns may help the clinician to suspect and recognize specific causes of disease. It is hoped that this review facilitates clinicians’ familiarity with these distinctive radiographic patterns.
Footnotes
- Correspondence: James K Stoller MD MSc FAARC, Education Institute, Cleveland Clinic Foundation, 9500 Euclid Avenue, Cleveland, Ohio, 44195. E-mail: stollej{at}ccf.org
The authors have disclosed no conflicts of interest.
- Copyright © 2023 by Daedalus Enterprises