Abstract
Early in the COVID-19 pandemic predictions of a worldwide ventilator shortage prompted a worldwide search for solutions. The impetus for the scramble for ventilators was spurred on by inaccurate and often unrealistic predictions of ventilator requirements. Initial efforts looked simply at acquiring as many ventilators as possible from national and international sources. Ventilators from the Strategic National Stockpile were distributed to early hotspots in the Northeast and Northwest United States. In a triumph of emotion over logic, well-intended experts from other industries turned their time, talent, and treasure toward making a ventilator for the first time. Interest in shared ventilation (more than one patient per ventilator) was ignited by an ill-advised video on social media that ignored the principles of gas delivery in deference to social media notoriety. With shared ventilation, a number of groups mistook a physiologic problem for a plumbing problem. The United States government invoked the Defense Production Act to push automotive manufacturers to partner with existing ventilator manufacturers to speed production. The FDA granted emergency use authorization for “splitters” to allow shared ventilation as well as for ventilators and ancillary equipment. Rationing of ventilators was discussed in the lay press and medical literature but was never necessary in the US. Finally, planners realized that staff with expertise in providing mechanical ventilation were the most important shortage. Over 200,000 ventilators were purchased by the United States government, states, cities, health systems, and individuals. Most had little value in caring for patients with COVID-19 ARDS. This paper attempts to look at where miscalculations were made, with an eye toward what we can do better in the future.
Introduction
The COVID-19 pandemic created stresses on the health care systems of the world not previously seen or perhaps even imagined. Nowhere was this more evident than in the ICU. Respiratory therapists (RTs) were front and center for the pandemic providing respiratory care to critically ill patients suffering from severe hypoxemic respiratory failure and hemodynamic instability. All this was complicated by the presence of a contagious respiratory pathogen that was easily transmissible and for which there were no vaccines or known treatments.1,2
Early in the pandemic the focus was squarely on ventilators. In the lay press and the medical literature there were questions of how many were needed, how many were available, and would these have to be rationed?3-5 In retrospect, this focus might have been unavoidable, but the response should be carefully examined. This paper attempts to look at what was done, where missteps were made, and how we must do better in the future.
Estimating Ventilator Needs
Following the H1N1 outbreak in the early 2000s, the Department of Health and Human Services embarked on a study in concert with the American Association for Respiratory Care (AARC) to determine the number of ventilators in United States acute care hospitals.6 In a survey of 4,305 hospitals accounting for 83% of United States intensive care beds, 52,118 critical care ventilators were owned. The study also counted noninvasive ventilators, portable ventilators, and pediatric devices. The total number of ventilators approached 100,000 devices.6 Importantly, the number of ventilators owned could be estimated from the number of ICU beds in a given hospital. Presciently, Rubinson et al6 noted that “positive-pressure ventilation devices alone do not ensure mechanical ventilation capability. Expert and experienced staff who know how to care for critically ill patients are essential.” The combination of ICU beds, critical care personnel, and ventilators is the basis for a successful response to mass casualty respiratory failure.7 Ventilator supplies alone do not confer critical care.
More recently, Tsai and colleagues8 evaluated ventilators owned by hospitals in the American Hospital Association annual survey. The goal of the study was to determine if there was an increase in the number of adult and pediatric ventilators across a range of United States facilities. The survey response rate was 59% and demonstrated an increase in the number of adult (30%) and pediatric ventilators (15%) owned during the COVID-19 pandemic.9 The response rate limits the findings somewhat, but this is the first paper to address the surge of ventilator purchases by hospitals during the pandemic.
In an accompanying commentary, Rubinson and others reinforced earlier statements. The focus on ventilation devices was flawed. Having a mechanical ventilator does not guarantee mechanical ventilation or critical care of which mechanical ventilation is only a component. In the absence of critical care staff, personal protective equipment (PPE), treatment space (often referred to as the ICU bed), and additional essential equipment (eg, intravenous pumps, physiologic monitors), ventilators are insufficient.10 Future investments by the federal government should focus on the entire critical care response (the space, the staff, and the supplies) not a single piece of equipment.
A discussion of predicted ventilator needs is not complete without referencing the pandemic within the pandemic, misinformation.11-13 In March of 2020 the New York Times reported that up to one million ventilators would be required.14 The governor of New York predicted he would need at least 30,000 ventilators (https://thehill.com/homenews/state-watch/489214-cuomo-says-ny-needs-30000-ventilators-pleads-with-feds-for-help/. Accessed December 6, 2022). Ranney and others15 commented that “No matter which estimate we use, there are not enough ventilators for patients with COVID-19 in the upcoming months.” The number of ventilators needed cannot be separated from the critical care capacity. This was known before COVID-19, and this is a lesson we should not relearn in the next pandemic.16
In fairness to all those involved, early in the pandemic, when so much was unknown, these projections appeared to have some basis in fact. The New York experience was fortunately not seen in every major city in the United States, nor was the impact in each region of the country simultaneous. High mortality rates and the regional surges of COVID-19 worked in concert to avoid a nationwide ventilator shortage.
The Strategic National Stockpile
Delivery of Existing Devices
At the beginning of the pandemic the Strategic National Stockpile (SNS) included approximately 18,000 ventilators, including the 2 legacy devices that were 20 years in storage, the Medtronic LP10 (Medtronic, Minneapolis, Minnesota) and the Zoll 754 Eagle (Zoll Medical, Chelmsford, Massachusetts). The original purchase included around 2,000 of each. In March of 2020, the number of these that remained functional were unknown. Even at peak performance, neither device could provide pressure control ventilation or pressure support and the inspiratory flow was limited to 60 L/min. Neither had the capability to monitor delivered flow or volume or display the waveforms.17 By current standards, it is doubtful what the utility of these devices would have been in treating severe hypoxemic respiratory failure and viral sepsis. To our knowledge, none of these devices were distributed during the COVID-19 pandemic.
The majority of ventilators in the stockpile were LTV 1200s (Vyaire, Mettawa, Illinois) purchased in and around 2011. The LTV 1200 is capable of volume and pressure control ventilation, pressure support including adjustments of rise time and flow termination, and a peak inspiratory flow of > 100 L/min. Importantly, the LTV 1200 is approved for patients weighing as little as 5 kg, lending to safe use in pediatrics. The ventilator includes a flow transducer integral to the circuit allowing the measurement of pressure, volume, and flow at the airway.17 LTV 1200s were sent to the Northeast (New York and New Jersey) as well as the Northwest (Seattle). Devices shipped to the Northeast were immediately put into use.
One concern of the SNS has always surrounded maintenance of the ventilators.18,19 This includes charging batteries, verifying functionality, and replacement of disposable components. Stockpile maintenance over time can exceed the original acquisition costs. This is not a trivial matter. Purchasing devices without a budget for maintenance is folly. Prior to the pandemic, maintenance contracts were in flux; and upon arrival, some devices were nonfunctional. This appeared to primarily be the result of battery failure.20 Battery discharge is an important concern. The ventilators could be connected to electrical power and operated in a given location. However, batteries either could not accept or maintain a charge. When transporting patients from the emergency department to the ICU, battery failure could occur in a few minutes, cease ventilation, and appear as ventilator failure.21
Importantly, during use, a number of concerns were identified. In these critically ill patients, often in isolation, it was difficult to see the ventilator settings from outside the room. Staff limited entry into rooms to avoid exposure. The lack of a waveform display was seen as a degradation in the standard of care.10,21 The LTV 1200 did not have the capacity to interface with the electronic medical record or central monitoring systems. The LTV 1200 had a monitoring screen available (LTM, Vyaire), but it was not part of the stockpile and had not been manufactured in over a decade. Of note, the group at The Center for Bits and Atoms at Massachusetts Institute of Technology (https://cba.mit.edu. Accessed December 6, 2022) was able to create a monitoring screen using a Raspberry Pi (https://www.raspberrypi.com. Accessed December 6, 2022) in a few days; but concerns over FDA approvals, sponsorship, and safety prevented any clinical application. Over time, as the paradigm of critical care changes, future purchases should assure the technology meets the needs of the patient and caregiver standards. Given this experience, purchasing ventilators with less functionality than the LTV 1200 should be avoided.
Acquisition of New Devices
In the late spring of 2020, the federal government set a goal for purchasing an additional 200,000 ventilators. Manufacturers from around the world were contacted regarding manufacturing capability. The federal government also enacted the Defense Production Act (DPA), encouraging automobile manufacturers to partner with ventilator manufacturers to help resolve supply chain issues and increase manufacturing capacity.22 We have previously discussed these purchases and issues in detail.21 A brief review is provided below.
In the waning months of 2020, the federal government procured 15 different ventilators ranging from ICU ventilators to a ventilator used to enable patients with chronic respiratory failure to ambulate. Table 1 lists the devices and manufacturers purchased. At present it is difficult to estimate the total number of devices purchased, but the devices manufactured under the DPA alone totaled 80,000 ventilators (Ventec V+Pro and Airon pNeuton A-E).23,24,21 As might be expected, the capabilities of the ventilators vary widely; and the groups in charge of purchases had no medical expertise, much less ventilator expertise. This was disappointing as minimum requirements for ventilators used for acute respiratory failure have been published.17,25 Perhaps as importantly, the focus was on the set parameters (tidal volume [VT], PEEP, breathing frequency). In critical care, monitoring and alarms are also crucial, as large numbers of patients were being cared for in isolation rooms equipped with negative-pressure exhaust. Limited alarms, too many patients for the usual staff ratios, behind closed doors with the added ambient noise were a bad combination for a ventilator with limited monitoring and alarms. In many cases, the volume of sales for a pandemic order were equivalent to a decade’s worth of sales in normal times. Suitability for critical care use is in fact not in the eye of the beholder, but the dollar values involved clearly stretched what manufacturers envisioned as a critical care ventilator capable of supporting patients with COVID-19.
Ventilators Procured by the Government to Support COVID-19
Of course, there is always the argument that simpler devices could be used for less sick patients, patients without COVID-19, or as transport ventilators. There will always be concerns of bias, and opinions will vary. One chief medical officer for a manufacturer recounted, “It’s better than nothing.”26 This is clearly not the standard for which ventilators should be procured. It’s important at this juncture to recalibrate our thinking on ventilators, how many are needed, and what functionality they should have. Table 2 compares what we would consider a portable ventilator for use in critical care, the Hamilton T1 (Hamilton Medical, Bonaduz, Switzerland); the LTV 1200, which was deployed in the pandemic and judged to have numerous shortcomings; the pNeuton A-E (Airon, Melbourne, Florida) manufactured under the DPA; and the SAVe II (AutoMedx, Addison, Texas). Side by side the comparisons are striking. As concerns regarding the utility of the LTV 1200 were known, the purchase of ventilators with capabilities far below the LTV 1200 was disappointing. From our standpoint, about half the ventilators that were purchased are ill-suited for use in critical care. In fact, we would encourage FDA to reconsider the definition of a critical care ventilator to make these distinctions clear even to the uninitiated. These are serious decisions that should be made by critical care teams who will care for these patients.
A Comparison of the T1 (Hamilton Medical, Bonaduz, Switzerland), LTV 1200 (Vyaire, Mettawa, Illinois), Airon pNeuton (Airon, Melbourne, Florida), and SAVe II (AutoMedx, Addison, Texas)
Going forward the federal government has serious stockpiling issues to address. Will all the ventilators purchased remain in the stockpile? The cost of yearly maintenance will be in hundreds of million dollars. Will certain devices with little clinical utility be disposed of or deployed in other nations? How will the Department of Health and Human Services train RTs and physicians to use 15 different ventilators? These decisions should be made with greater forethought than was undertaken for the initial ventilator purchases.
Emergency Use Authorization
Early in the pandemic, the FDA provided notice of Emergency Use Authorization (EUA) for devices and drugs to the treatment of COVID-19 (https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization. Accessed December 6, 2022). This included a group of devices under the title of “Ventilators and Ventilator Accessories.” This category included ventilators, resuscitators, filters, humidifiers, splitters, helmets, masks, and CPAP generators (https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/ventilators-and-ventilator-accessories-euas#ventilators. Accessed December 6, 2022). This is an impressive list and is shown in Table 3. Note that a couple of the ventilators purchased for SNS are in this list, and neither is identified as a critical care ventilator. Other EUA designations allowed for the use of anesthesia ventilator in the ICU and noninvasive ventilation (NIV) ventilators used for invasive ventilation.
Emergency Use Authorizations for Ventilators and Ventilator Accessories
Anesthesia Ventilator Use in the ICU
A number of authors (we were among them) had previously suggested that the operating theater or the transfer of anesthesia workstations to the ICU represented a ready source of ICU style ventilators to meet a surge in cases requiring ventilatory support.6,17,25,27 The anesthesia workstation is a multicomponent device that includes a ventilator, physiologic monitor, oximeter, capnograph, and oxygen analyzer. Ventilators integral to the anesthesia workstation range from relatively simple to ICU functionality.28 The FDA authorized anesthesia workstation use in the ICU under the EUA.
Use of the anesthesia ventilator at the bedside proved to be a far greater challenge than anticipated.28-35 The presence of the circle system for rebreathing during anesthesia was foreign to ICU teams, and created problems with excess moisture in the ventilator circuit and occlusion of heat and moisture exchangers (HMEs).32,34-35 Anesthesia workstations are meant to be attended by anesthesia personnel (certified registered nurse anesthetists or anesthesiologists) for cases < 24 h. As a consequence, alarm volumes are low compared to ICU devices, and the anesthesia workstation had to be disconnected from the patient and undergo a check-out procedure every 24 h. Table 4 lists some common problems encountered with anesthesia ventilator use in the ICU.
Challenges in Using Anesthesia Ventilators in the ICU
Most of the reports regarding anesthesia ventilator use in the ICU were anecdotal or small case series. However, Bottiroli et al35 retrospectively compared outcomes of subjects with COVID-19 ventilated with an ICU ventilator to those ventilated with an anesthesia ventilator. The study has a number of limitations related to different care sites for subjects (operating theater vs ICU), and there were far fewer subjects ventilated with anesthesia ventilators than subjects ventilated with ICU ventilators (17% vs 72%). Mortality rate in the group on anesthesia ventilators was 70% versus 37% for subjects on an ICU ventilator in the ICU. Despite the study’s limitations, this report should give us pause regarding the use of anesthesia devices in the ICU. More importantly, if anesthesia ventilators are to be part of a surge strategy, known challenges of anesthesia ventilator use should be addressed prior to an event with education of ICU staff and hands-on practice.
Splitters
Shared ventilation has been postulated as a solution for mass casualty respiratory failure in the past, with little to no evidence of clinical use or utility.36,37 Ventilation of 4 patients simultaneously stretches credulity, even aside from the pragmatic issues of placing 4 patients close enough to one another to connect to a single ventilator during a pandemic driven by a contagious respiratory virus, its use is potentially life threatening to all involved. Proponents of shared ventilation mistake a physiology problem for a plumbing problem.38
However, early in the pandemic a number of “splitters” were authorized by the FDA under the auspices of the EUA (https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/ventilators-and-ventilator-accessories-euas#tubing. Accessed December 6, 2022). In early descriptions of shared ventilation, the circuits to allow 2–4 patients to be connected to a single ventilator were cobbled together from spare parts including T-pieces and connectors to allow a reservoir bag to attach to a ventilator.36 In an effort to provide connectors that were streamlined, provided laminar flow, and were readily available, a number of groups 3D printed or molded splitters. Shared ventilation is discussed separately, but this is another example where sincere effort was wasted on an unproven and potentially dangerous therapy.
In several cases, the delivery of these splitters was heralded as a solution for the upcoming ventilator shortage. They were not. As of this writing, 9 of the splitter devices authorized under the EUA have been rescinded.
Noninvasive Ventilators for Invasive Ventilation
A number of ventilators commonly used for NIV have approval for use during invasive ventilation. The FDA approved ventilators for invasive ventilation that were traditionally used for NIV but were classified as “continuous minimal ventilatory support, facility use.” More simply these were not intended for continuous life support. NIV devices primarily differ from traditional ICU ventilators in that these are meant to operate in the face of a leak (intentional or at the interface). However, some NIV ventilators have significantly greater monitoring and alarm capabilities than some of the emergency ventilators that were acquired.
NIV use in hypoxemic respiratory failure remains controversial. A number of studies have shown increasing failure rates with worsening arterial oxygenation that is also associated with excess mortality.39-41 Data from the LUNG SAFE study found an NIV failure rate of 47% in severe ARDS (PaO2/FIO2 < 150 mm Hg) and an increased ICU mortality (odds ratio 1.45).40 More recently, Menga and others41 found that NIV in COVID-19 was associated with a 2-fold greater risk of failure. As in previous trials, greater severity of illness, more significant hypoxemia, and elevated serum lactate dehydrogenase were associated with greater NIV failure risk.42
Early in the pandemic, NIV was often avoided owing to a concern of caregiver exposure to aerosol-generating procedures.43 A number of recent studies have demonstrated that NIV is in fact not an aerosol-generating procedure, and the risk is no greater than with any patient with an intact upper airway.44,45 However, PPE use by caregivers is mandatory, and NIV can act to spread particles expelled during a cough.45
NIV and bi-level devices used during either invasive or NIV modes may use an external exhalation valve or a passive circuit with a fixed leak. These devices are not commonly equipped with humidifiers or expiratory filters. With concern for caregiver protection, filters were added to the circuit at the leak port, in the expiratory limb, at the airway, or in more than one site.46
Patout and others46 evaluated the impact of filters on NIV device performance using 8 different circuit configurations. These included single- and dual-limb circuits, passive and active exhalation valves, and both helmet and mask interfaces. This bench model evaluated the impact of filters on triggering, delivered pressure and volume, work of breathing trigger delay, and pressure-time product to trigger the device. They found statistically significant differences in all the measured parameters following placement of a filter. The authors concluded that a dual-limb circuit with an oronasal mask performed best, and the worst performance was seen with the helmet and dual-limb circuit. They recommended that a dual-limb circuit and oronasal mask be used if possible.46
In a subsequent study, Tolson and colleagues47 performed a similar bench evaluation using 4 different ventilators and 3 different circuit configurations. Filters were placed in different locations within the ventilator circuit. In general, filters reduced the VT, peak flow, and delivered airway pressure. Triggering was negatively impacted in 3 of the 4 ventilators studied, and one ventilator failed to trigger with a filter between the interface and the exhalation port. This position is necessary to filter expired gases but adds resistance that is not accounted for by the ventilators’ triggering alogorithm.47 Adjustment of the trigger threshold can overcome this problem.
Repurposing NIV devices for invasive ventilation represents a readily available method for augmenting ventilator capacity. These devices can also be used as intended for NIV, and some have the ability to provide high-flow oxygen therapy. However, adaptation of NIV or bi-level devices for invasive ventilation with the addition of breathing circuit filters alters device performance. Off-label applications with bedside modifications can have unintended consequences.48,49
Shared Ventilation
Shared ventilation, sometimes referred to as multiplex ventilation, describes the use of a single ventilator to support more than one patient.38 Although often attributed to a simple descriptive study published in 2006,36 the first description of using a single ventilator for 2 subjects was described by Sommer et al50 in 1994. Neither system was ever tested on patients, and the paper by Neyman and Irvin36 simply observed the rise and fall of 4 rubber test lungs connected to a ventilator with a set VT of 2 L via a series of T-connectors. As previously noted, this was not a scientific evaluation but simply a “tinker toy” exercise.51
In 2003, Branson and colleagues52 demonstrated a plethora of problems associated with shared ventilation using a bench model of 4 patients and measurements of airway pressure, volume, and flow with laboratory instruments. Results from this study were all easily predictable from mathematical models. Changes in compliance resulted in variable VT delivery and unequal changes in end-expiratory lung volume. Perhaps as importantly we noted practical issues. With 4 adult circuits, the ventilator could not pass the self-test as tubing compliance was out of range and leaks were inevitable. Operation of the ventilator and volume measurements were then suspect. Triggering was negatively impacted, and spontaneous breathing in one simulator resulted in “sharing” of gas between circuits. Catastrophic failure (occluded artificial airway, circuit disconnection) in one test lung resulted in failure of ventilation in the other four. Finally, a single measure of pressure and volume could be made with no distinction as to individual values. These considerable shortcomings would seem to have closed the door on shared ventilation for multiple patients.52
Unfortunately, early in the COVID-19 pandemic, an ill-advised social media video proclaimed an easy method for saving lives by placing 4 patients on a single ventilator. In short order the video had over half a million views, and at this writing views remained just under 1 million views, despite multiple requests to take down the post. We ask that readers understand our reticence to provide the URL and further spread misinformation. At its worst, this video allowed one individual to encourage nearly a million individuals (we can’t know how many viewers were health care workers) to use an unapproved, off-label technique that could harm millions. The initial response by critical care societies was a statement discouraging shared ventilation—particularly 4 patients at a time—and encouraging consideration of more reliable alternatives including the use of NIV, CPAP, and portable ventilators.53
In the ensuing months, a number of groups developed systems to help overcome the naïve approach of simply adding T-connectors. These modifications included pressure-limiting valves, individual PEEP valves, one-way valves to reduce cross-contamination, additional pressure and volume monitoring, flow restrictors, proportioning valves, and further circuit modifications.38,54-68 Appropriately, most serious authors realized the futility of supporting 4 patients with a single ventilator and focused on 2 patients per ventilator.
Nearly all of these studies evaluated the improvised systems in a bench model. Most agree that paralysis and deep sedation are required to prevent patient triggering. Allowing the patients to breathe grants one patient the ability to set the rate for all involved, increases the risk of cross-contamination, and undoubtedly leads to asynchrony. In each case, the systems were primarily tested with a single ventilator. This included anesthesia ventilators, critical care ventilators, and portable ventilators. This represents an additional shortcoming of shared ventilation. Mechanical ventilators are complex devices with major to subtle differences in breath delivery algorithms and position of pressure and volume sensors as well as anti-asphyxia valves. Simply because a system for shared ventilation between 2 patients functions without alarms or failures with one ventilator does not mean it will function with other ventilators.38,58,62-64
Published reports of shared ventilation use in human subjects pale in comparison to the number of systems devised to undertake the endeavor. To our knowledge 3 clinical reports of shared ventilation of pairs of patients have been published. The shared world published experience of shared ventilation is limited to 10 patients.69-71
Raredon and colleagues69 described a system for shared ventilation using modified PEEP valves in the inspiratory and expiratory limbs of each circuit to control peak and end-inspiratory pressures. They described ventilation of a pair of subjects with COVID-19 with pulmonary compliances of 22 mL/cm H2O and 26 mL/cm H2O, respectively, using pressure control ventilation. They observed the development of 2–3 cm H2O of auto-PEEP, likely related to increased resistance of the circuit and a small increase in PaCO2 in each subject. Increased compressible volume of the circuit and a fall in effective VT could explain the change in CO2 elimination. Gas exchange was maintained over a 4-h time frame, and no adverse events were identified.
Levin et al70 described the ventilation of 2 pairs of subjects with COVID-19 for a 1-h duration. Subjects were matched for current conventional ventilator settings including PEEP, FIO2, and breathing frequency. Arterial blood gases were measured every 30 min, and VT was independently adjusted using flow control valves during pressure control ventilation. Despite disparate lung mechanics between the subjects, gas exchange was maintained.
The largest published clinical use of shared ventilation was described by Beitler and colleagues.71 This experience included ventilation of 3 pairs of subjects with COVID-19 ARDS for a period of 48 h. Approval was sought from the State of New York, the health system, and the institutional review board prior to attempts to approach subjects. Following approval, informed consent was obtained from the subjects’ authorized representatives. Initially, subjects were matched by driving pressures measured during standard ventilation. No circuit modifications were made beyond a T-piece to split the circuits; however, an independent respiratory monitor was used for each subject to continuously monitor airway pressure, volume, flow, and end-tidal CO2. Neuromuscular blockade was undertaken, and all ventilators were operated in pressure control.
The first set of subjects were ventilated with an anesthesia ventilator. A number of complications were reported including excess humidity, rainout, and the resultant increased resistance of HMEs. Additionally, frequent exhaustion of the CO2 absorbent occurred as minute ventilation and CO2 production of critically ill subjects with viral sepsis and ARDS far exceeded that typically seen in the operating room. When HMEs collected moisture, resistance increased with a resultant fall in VT and rise in PaCO2 during pressure ventilation.
The remaining subject pairs were ventilated with ICU ventilators without incident, although increased HME resistance and the attendant consequences were seen due to thick, copious secretions. A major limitation of shared ventilation is the need for frequent blood gases to determine ventilation and oxygenation. Beitler et al71 concluded that shared ventilation was feasible but that the additional monitoring and surveillance were counterproductive in a critical care surge of too many patients and too few caregivers.
They emphasized that institutional approval of a rigorous clinical protocol, informed consent from the patients’ surrogates, carefully matching of patient pairs, and the use of continuous neuromuscular blockade were critical to success. The coauthors of this paper included one of the authors of this paper (RB) as well as several other well-known RTs (Rich Kallet, Dean Hess, Bob Kacmarek). Several of these authors also contributed to the critical care societies’ statement warning against shared ventilation use. This is a bit of a contradiction. The critical care societies clearly stated that ventilation of 4 patients should not be attempted and discouraged use in a pair of patients. During the height of the pandemic in New York City, Beitler and colleagues contacted each of the RTs who were eventually coauthors. The protocol, mitigation strategies, and ethical issues surrounding shared ventilation were adjudicated by the group over a period of several days to optimize the chance of success and reduce risks. The decision was made that attempting shared ventilation when there was no choice was undesirable but an early attempt to determine feasibility was warranted. And whereas the use was a success, the limitations were quickly noted, and no further attempts at shared ventilation were made. The lion’s share of the credit for this herculean effort and the knowledge gained should be rightfully attributed to Dr Beitler and his colleagues at Columbia University.
De Novo Ventilator Designs
With daily emphasis on the impending ventilator shortage saturating the nightly news and social media, individuals and corporations with engineering experience and time on their hands from business shutdowns turned their attention to assist in solving the problem. From a societal standpoint, individuals who dedicated their time, talent, and treasure toward developing ventilator technology represent exemplary human qualities. As in previous discussions, what constitutes a mechanical ventilator and the requirements of these ventilators were ill-defined and for many groups unknown. Ventilators were made by Fitbit, Virgin, NASA, and Dyson. But expertise in wearables, flight, and vacuum technology did not translate well to development of a life-support device.
Mechanical ventilators are sophisticated devices with complex software, gas delivery systems, monitoring, and alarms that often take years to develop. A typical 510(k) FDA submission for an ICU ventilator often requires as many as 10,000 pages. These facts create a conundrum. Creating a device with the sophistication required to care for patients with hypoxemic respiratory failure was beyond the time constraints to meet demand and the capabilities of the designers. Ventilators that can be developed in a short time frame don’t have the sophistication or safety required for these patients.
Making a Ventilator for the First Time
First-time ventilator developers were spurred on by anticipated needs and the desire to provide solutions. There were also sponsored contests to encourage developers to bring technology to bear. In one instance, the project was known as the CoVent-19 Challenge and operated by a group out of the Massachusetts General Hospital (https://advances.massgeneral.org/research-and-innovation/article-external.aspx?id=1095. Accessed December 6, 2022). The team consisted of 12 residents from the Department of Anesthesia, Critical Care and Pain Medicine and a number of external consultants serving as judges. One of the judges was Robert Kacmarek PhD RRT FAARC, and another one of the authors of this paper (RB). The CoVent-19 Challenge was a crowdsourcing solution to encourage the global medical community to develop rapidly deployable designs to increase access to mechanical ventilation. The sponsors of the CoVent-19 Challenge were Stratasys, Ximedica, Hackers/Founders, and Valispace (https://grabcad.com/challenges/covent-19-challenge-round-1. Accessed December 6, 2022). There were $10,000 in prizes.
There were 208 entries including NIV devices, CPAP devices, bag squeezers, and devices to ventilate multiple patients. The winning design was the SmithVent developed by a team of Smith College engineering alumni and colleagues (https://grabcad.com/library/smithvent-1. Accessed December 6, 2022). The device focused on readily available parts versus any novel design. To our knowledge the device was never manufactured or deployed.
The Hack-a-Vent Challenge was part of the Vulcan-5 Ventilator Project sponsored by the United States Special Operations Command (https://www.vulcan-v.com. Accessed December 6, 2022). The program accepted 172 designs, triaged this down to 5 candidates, and in a period of 8 weeks moved from launch of the challenge to EUA submission. Variability in device design and sophistication were pronounced. One device, CorVent (Coridea, New York, New York) was awarded an EUA but to our knowledge was never used in patients. Figure 1 is the internal mechanism for 2 Hack-a-Vent style systems. Both use a piston, and the device on the left uses a windshield wiper motor to control the breathing frequency. Airway pressures are controlled by external PEEP valves (spring-loaded disks) or water columns.
Two first-time ventilators developed for COVID-19. Both use a piston for gas delivery, and the device at left uses a windshield wiper motor to control the breathing frequency.
We applaud the efforts and ingenuity of these groups and their desire to come to the aid of patients and an overwhelmed health system. However, these devices were not a viable solution. None were deployed. With deference to these efforts and the spirit in which they were undertaken, in future pandemics if you are inclined to build a ventilator for the first time, don’t!
Bag Squeezers
Clinicians who have studied and prepared for mass casualty respiratory failure are all familiar with what is widely regarded as the first such event, the polio epidemic in Denmark. As it relates to mechanical ventilation, Lassen and colleagues72 pivoted from the use of a handful of negative-pressure ventilators to tracheostomy and manual ventilation. Initially, manual ventilation was provided with a non–self-inflating bag, including a low flow of oxygen and CO2 absorber. The CO2 absorber allowed conservation of oxygen, in that time piped in oxygen was non-existent, and provided heat and moisture to the inspired gas. Figure 2 depicts the progression by Lassen and others; this system included a heated humidifier and non–rebreathing valve, but the power remained human. In this instance, medical students providing manual ventilation in 4-h shifts.72
Early use of manual ventilation during the polio epidemic in Denmark. The medical student provides the power for ventilation using a non–self-inflating bag. An early electric humidifier is also shown. From Lassen HCA, Management of life-threatening poliomyelitis. Copenhagen 1952–1956, with a survey of autopsy findings in 115 cases. Edinburgh: ES Livingstone;1956:65, with permission.
During the polio epidemic 1,500 students were pressed into service as human ventilators, and in a tribute to this intervention, the mortality rate fell from 87% with negative-pressure ventilators to < 40% with manual support. As positive-pressure ventilators were rapidly developed, Ibsen and colleagues72 continued to prefer manual ventilation, suggesting it conferred some advantages to mechanical respirators. Critical to this discussion and the success of manual ventilation is remembering that these patients were ventilated for muscle paralysis, not lung injury. In fact, Lassen, Ibsen, and others may have introduced the modern concept of intensive care as the site where patients requiring mechanical ventilation were housed. Importantly, their experience never included ventilatory support of patients with primary lung pathology. COVID-19 pneumonia, ARDS, and viral sepsis have little in common with polio.72
Manual ventilation has often been touted as a potential solution for mass respiratory failure events, but manpower shortages and caregiver exposure preclude use as anything but a temporizing measure. In addition, providing a consistent VT, FIO2, PEEP, and flow is impossible. Matching patient effort and monitoring are also a concern. To overcome the manpower limitation, the Massachusetts Institute of Technology (MIT) ventilator was described in 2010.73 The claim was that the system was “low cost, low power, portable ventilator technology” able to be used by novices.
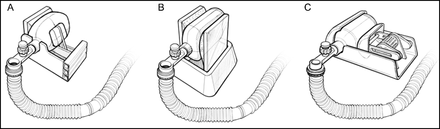
At its simplest description, the MIT ventilator is an automatic “bag squeezer.” During COVID-19, the designs for “bag squeezing” proliferated. These included devices that had any number of methods for compressing the resuscitator from compressing it between 2 plates, compression with a piston, or in any number of squeezing mechanisms. Monitoring was added in some cases, primarily airway pressure and remote control and monitoring possible in some designs.74-89 Figure 3 depicts generic examples of bag-squeezing mechanisms.
Methods for squeezing a manual resuscitator.
To our knowledge none of the bag-squeezing devices were used to support patients with COVID-19. As with other proposed solutions, the development of rapidly deployable, easy to manufacture, and easy to use devices fails to address the issue. Mechanical ventilation is complex and requires not only the ability to trigger on patient effort but delivery of sufficient flow to meet demand, control airway pressure, and VT for safety. Monitoring and alarms are limited with these devices, which is a major concern. Simplicity is perhaps a disadvantage, and training staff who have never seen these device raises concerns. Ultimately the overarching theme persists. Mechanical ventilators are just one component of critical care; ventilators used in this situation should be critical care ventilators.
Open-Source Ventilators
A number of experienced groups developed devices with the focus on readily available components and simplicity. Many included the design and materials as open source. The idea being that using parts unaffected by the supply chain problems brought on by COVID-19 and open-source documentation these devices could be manufactured locally.90-97
As with other first-time ventilators, none of these reports include clinical use or testing during the pandemic. These projects were great experiences for engineering and medical teams to work in tandem for the betterment of society. In our opinion, these are not an answer to a surge of patients with ARDS requiring mechanical ventilation.
Practical Solutions
After almost 3 years of the COVID-19 pandemic and multiple proposed ventilator solutions ranging from bad to middling ideas, we firmly believe attention needs to turn toward solutions that include the provision of mechanical ventilation as part of a critical care strategy. This includes ventilators designed for critical care, support for adequate respiratory therapy staffing, and all the required ancillary equipment. In the interim, there are some advances that could be implemented using existing equipment with regulatory changes allowing care of patients in isolation more effectively. We also believe there are better logistic solutions for having a readily available source of critical care ventilators.
Monitoring and Controlling the Ventilator Outside the Room
Early in the pandemic, in the presence of an unknown respiratory pathogen and no known treatments, lack of vaccines, and no innate immunity of caregivers, isolation of patients was mandated. The requirement for extensive PPE donning to respond to emergencies in critically ill patients with sepsis and ARDS was a standard of care. More routine room entry was reduced to save on PPE and reduce caregiver exposure.
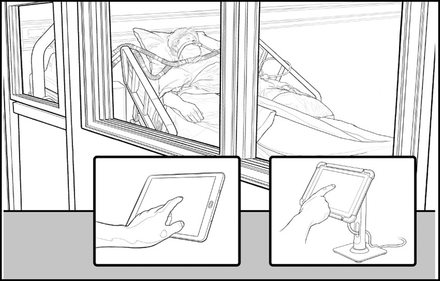
A number of critical care ventilators commonly used in the ICU have control panels mounted on the device, but these frequently have a tether wound behind the panel which can reach as long as 8 feet. These panels can be mounted outside the room where simple interactions including increasing FIO2 or silencing alarms could be accomplished without PPE use or caregiver exposure. We observed this practice during the pandemic, but there is no published literature available. Figure 4 demonstrates this idea.
Depiction of the ventilator control panel placed outside the room (right) or wireless control of the ventilator (left).
The technology to monitor the ventilator and make changes on the ventilator remotely has been available for decades. Concerns over cybersecurity and regulatory requirements have prevented this concept from being commercialized. In our opinion, the ability to increase FIO2 from outside the room to correct hypoxemia, silence alarms, and monitor waveforms could provide advantages during the next pandemic. This would not require an EUA, open source, or first-of-a-kind designs. Figure 4 depicts this simple concept that could save money, reduce exposure of the ICU staff, and allow faster response to changes. These thoughts require confirmation in clinical experience.
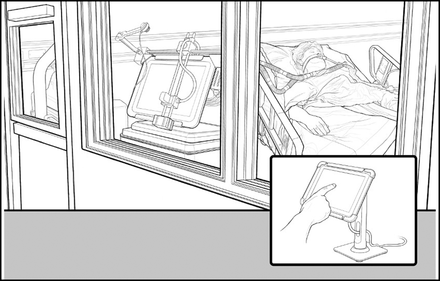
A group of engineers from Johns Hopkins also recognized the advantages of making changes on the ventilator remotely. They developed a robotic system mounted to the display panel of the ventilator and operated from outside the room. They noted PPE and time savings for RTs by eliminating the need to don and doff PPE.98 Figure 5 depicts this concept.
Robotic control of ventilator settings from outside the patient’s room.
These authors should be congratulated for recognizing a problem and using their expertise to solve it. This solution, however, falls under the category of needless complexity. Systems would have to be calibrated for each brand of ventilator and for each page of the touch-screen interface. What remains apparent is that a method to control and monitor the ventilator from outside the room could be valuable in the next infectious pandemic.
Automated Ventilation
Artificial intelligence and automated control of oxygenation and ventilation might play a similar role during a pandemic, making changes within a predefined framework to improve patient safety and reduce the need for entering the room.99 In the United States automated control of PEEP and/or FIO2 has not been approved. This is an area where an EUA might have proven useful.
Automated systems are available outside the United States, and undoubtedly these modes of ventilation were used. Buiteman-Kruizinga et al100 evaluated automated control of minute ventilation, PEEP, and FIO2 (INTELLiVENT, Hamilton Medical) in a convenience sample of subjects with COVID-19. In a crossover study compared to conventional mechanical ventilation, automated support was associated with lower driving pressures and mechanical power. The clinical importance of these changes is unclear. The availability of automated control might provide similar advantages to control of the ventilator outside the room. Research in future pandemics should evaluate the utility of automated ventilation and oxygenation in facilitating care.
Rethinking the Stockpiling of Ventilators
Since 2000, the SNS has housed ventilators at various sites around the country. The conventional paradigm is to make a large purchase ∼2,000 ventilators and house these devices over an ill-defined period of time. There are a number of concerns with this paradigm. First, the ventilators must be useful in an ICU and undergo expensive preventive maintenance.18 Over time the performance of devices may degrade and as with the case of the LP10 and Impact 754, housed for over 20 years, no longer be capable of providing the standard of care 2 decades into the future.
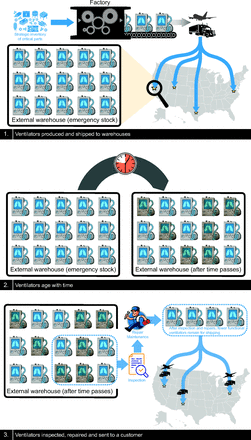
As noted previously, even the LTV 1200s were considered to have significant limitations after 10 years, and maintenance issues rendered many devices either poorly operational or nonfunctional. Figure 6 depicts the current paradigm for ventilator stockpiling.
The current paradigm for stockpiling mechanical ventilators.
We believe the stockpiling paradigm should be reconsidered. To begin with, only ventilators capable of caring for critically ill patients should be acquired. The most recent purchases acquired over 70,000 ventilators ill-suited to critical care.21 Kaliya-Perumal101 and others have suggested rethinking stockpiles, moving from physical to a virtual stockpile. In this model instead of acquiring large caches of ventilators, which carry an large upfront investment and ongoing maintenance, a virtual stockpile has little initial cost but relies on rapid manufacturing by ventilator companies. In the latter case, if 20 years pass between events, maintenance costs and depreciation of devices are alleviated. This model would, however, increase the time until device delivery.
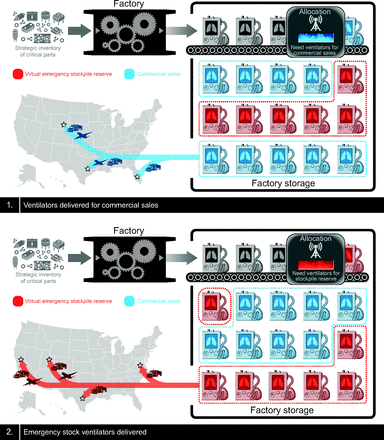
We are not logisticians, but postulate there is a possible model that prevents excessive maintenance costs and guarantees only ICU-capable ventilators. The government could contract with United States manufacturers to maintain excess inventory within their facilities beyond current standards. This would require contracting with multiple manufacturers; a single entity would be insufficient. As ventilators are manufactured and stockpiled in a warehouse, commercial sales would be provided from this inventory. As the inventory dwindles, additional ventilators are manufactured to replace those sold. In this way, the “virtual stockpile” is always refreshed and maintenance and storage costs traded for additional inventory. As ventilator models are upgraded, the stockpile would be upgraded as well. Figure 7 describes this concept.
A proposed alternative to traditional ventilator stockpiling.
A Note on Ventilator Donations to Low- and Middle-Income Countries
During COVID-19 as open-source ventilators, bag-squeezers, and first-of-a-kind devices were developed, a number of investigators quickly recognized the complexities of building a ventilator far exceeded the expertise of the team. As we have shown, an ICU ventilator is a complex life-support device with a dizzying array of requirements. At this point in development, realizing FDA approval was nearly impossible; the goal changed to providing ventilators to low- and middle-income countries.
The race to send ventilators to a host of low-resource nations was the last of the missteps in the COVID-19 ventilator story. A number of physicians working in Africa cautioned against this practice.102,103 In the absence of infrastructure (reliable electricity, oxygen, clean water), ICU capacity, and ICU trained staff, 10,000 ventilators would not have impacted patient outcomes.104,105 These are classic mistakes where wealthier nations presume to know the needs of low- and middle-income countries without asking.
Summary
COVID-19 stressed the health care system in the United States and around the world. Mechanical ventilators became the poster child for the pandemic and garnered attention far beyond their importance. Prkachin106 described the “reign” of the ventilator in COVID-19 and reviewed the history of ventilators in a pandemic. Several other authors96,107,108 have called for a reevaluation of the response to a presumed ventilator shortage and realignment with a response that focuses on providing critical care services. Key among these priorities is a trained, protected critical care staff. We have tried to be direct regarding the mistakes surrounding the ventilator mania during COVID-19. We hope this review will provide some food for thought on how to improve going forward.
Acknowledgment
The authors are grateful to Konrad Solarewicz for his expert creation of the figures representing the concepts in this manuscript.
Footnotes
- Correspondence: Richard D Branson MSc RRT FAARC, University of Cincinnati, 231 Albert Sabin Way, ML #0558, Cincinnati, OH 45267. E-mail: richard.branson{at}uc.edu
↵† Deceased.
Mr Branson discloses relationships with Daedalus Enterprises, Inogen, Lungpacer, Ventec Life Systems, and Zoll Medical. He is Editor-in-Chief of Respiratory Care.
Mr Branson presented a version of this paper as part of the New Horizons Symposium: COVID-19 Lessons Learned at AARC Congress 2021 LIVE!, held virtually December 3, 2021.
- Copyright © 2023 by Daedalus Enterprises
REFERENCES
- 1.↵
- 2.↵
- 3.↵
- 4.
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.
- 30.
- 31.
- 32.↵
- 33.
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.
- 56.
- 57.
- 58.↵
- 59.
- 60.
- 61.
- 62.↵
- 63.
- 64.↵
- 65.
- 66.
- 67.
- 68.↵
- 69.↵
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.↵
- 75.
- 76.
- 77.
- 78.
- 79.
- 80.
- 81.
- 82.
- 83.
- 84.
- 85.
- 86.
- 87.
- 88.
- 89.↵
- 90.↵
- 91.
- 92.
- 93.
- 94.
- 95.
- 96.↵
- 97.↵
- 98.↵
- 99.↵
- 100.↵
- 101.↵
- 102.↵
- 103.↵
- 104.↵
- 105.↵
- 106.↵
- 107.↵
- 108.↵