Abstract
BACKGROUND: During noninvasive ventilation (NIV) of COPD patients, delayed off-cycling of pressure support can cause patient ventilator mismatch and NIV failure. This systematic experimental study analyzes the effects of varying cycling criteria on patient-ventilator interaction.
METHODS: A lung simulator with COPD settings was connected to an ICU ventilator via helmet or face mask. Cycling was varied between 10 and 70% of peak inspiratory flow at different breathing frequencies (15 and 30 breaths/min) and pressure support levels (5 and 15 cm H2O) using the ventilator's invasive and NIV mode with and without an applied leakage.
RESULTS: Low cycling criteria led to severe expiratory cycle latency. Augmenting off-cycling reduced expiratory cycle latency (P < .001), decreased intrinsic PEEP, and avoided non-supported breaths. Setting cycling to 50% of peak inspiratory flow achieved best synchronization. Overall, using the helmet interface increased expiratory cycle latency in almost all settings (P < .001). Augmenting cycling from 10 to 40% progressively decreased expiratory pressure load (P < .001). NIV mode decreased expiratory cycle latency compared with the invasive mode (P < .001).
CONCLUSION: Augmenting the cycling criterion above the default setting (20–30% peak inspiratory flow) improved patient ventilator synchrony in a simulated COPD model. This suggests that an individual approach to cycling should be considered, since interface, level of pressure support, breathing frequency, and leakage influence patient-ventilator interaction and thus need to be considered.
- noninvasive ventilation
- NIV
- patient-ventilator interaction
- cycling
- COPD
- chronic obstructive pulmonary disease
Introduction
Noninvasive ventilation (NIV) is the main therapy for respiratory failure due to exacerbations of COPD.1 A ventilator mode widely used for NIV is pressure support ventilation.2 In pressure support ventilation, inspiration is marked by a change of flow or pressure generated by the patient's inspiratory effort, detected and supported by the ventilator (triggering).3 Inspiratory pressure assistance decreases during the inspiration and is stopped at a predetermined percentage of peak flow, which is called cycling.4 When cycling is inefficient, it can cause patient-ventilator mismatch. Poor patient ventilator synchrony is common and is associated with increased work of breathing, discomfort, prolonged mechanical ventilation, and NIV failure.5,6
Premature cycling leads to periods of unassisted breathing and increased work of breathing.7 Furthermore, persistent inspiratory effort after pressure support ceases can lead to double-triggering,7 resulting in discomfort, elevated tidal volumes (VT), and volutrauma.8 Cycling after the end of inspiratory effort shortens the expiratory time, causing intrinsic PEEP9 and leading to increased work of breathing during triggering of the next breath.10,11
In former generations of ventilators, cycling criteria were fixed, usually to 25% of peak inspiratory flow.8 Lack of synchronization is common with this setting.4,5,9–11 This problem is highlighted in patients with COPD4,12 due to the long time constant.9–11 In modern ventilators, cycling can be adjusted.4 As has been shown for invasive ventilation, adjustment of cycling criteria improves patient ventilator synchronization and reduces intrinsic PEEP.13,14 However, studies assessing cycling in depth15 are lacking. The purpose of this lung model study with COPD settings was to systematically investigate how patient ventilator synchronization during NIV with 2 different interfaces (face mask and ventilation helmet) can be improved by adjusting cycling.
QUICK LOOK
Current knowledge
Patient-ventilator asynchrony during noninvasive ventilation (NIV) is most commonly related to missed triggers and delayed cycling associated with leaks. Poor patient-ventilator synchrony is common and is associated with increased work of breathing, discomfort, prolonged mechanical ventilation, and NIV failure.
What this paper contributes to our knowledge
In a lung model simulating NIV in a COPD patient, synchrony was improved when the flow cycle criteria ranged between 30 and 70% of peak flow. Increasing flow cycle criteria above the default level is recommended, as long as parameters suggesting delayed cycling are detectable.
Methods
A lung simulation device (ASL 5000, IngMar Medical, Pittsburgh, Pennsylvania), capable of simulating breathing, was connected to a dummy-head (Airway Management Trainer, Laerdal Medical, Stavanger, Norway) and ventilated by a standard ventilator (Servo-i, Maquet Critical Care, Solna, Sweden) via face mask (Hospital NV full face mask, ResMed, Bella Vista, Australia) or ventilation helmet (4VentNIV, Rüsch Medical, Kernen, Germany). An opening was created in the interfaces, which could be opened to simulate standardized leakage. All other leakage was barred by gluing the NIV interface to the dummy, and all settings with leakage were evaluated in the ventilator's NIV and invasive modes. Data were recorded by pressure and flow sensors placed in the respiratory circuit.
COPD settings were based on recently published data.16 Resistance and compliance were simplified to be constant. The following parameters were set (see the supplementary materials at http://www.rcjournal.com): 90 mL/cm H2O of compliance; 15 cm H2O/L/s of resistance; 8 cm H2O of inspiratory effort.
Breathing frequency of the lung simulator was set to 15 and 30 breaths/min. Based on these settings, inspiratory time (15 breaths/min: 1.331 s; 30 breaths/min: 0.711 s), expiratory time (15 breaths/min: 2.669 s; 30 breaths/min: 1.289 s), the resulting inspiratory portion of the total breathing time (inspiratory time/total breathing time) (15 breaths/min: 0.33; 30 breaths/min: 0.36), and airway occlusion pressure at 100 ms (15 breaths/min: 1.9 cm H2O; 30 breaths/min: 3.5 cm H2O), were varied during simulated breathing at the different frequencies in order to achieve a realistic simulation. PEEP was set to 7 cm H2O; the steepest pressure rise time (0 s) and maximum trigger sensitivity that did not lead to auto triggering were chosen.
Pressure support was set to 5 and 15 cm H2O. Cycling was set to 10, 20, 30, 40, 50, 60, and 70% peak flow, and these were partially grouped as low (10 and 20%), medium (30, 40, and 50%), and high (60 and 70%) cycling criteria.
Parameters measured (Fig. 1) were asynchrony index (non-supported inspirations/inspiration efforts × 100),6 double-triggering-index (double-triggering/inspiration efforts × 100), expiratory cycle latency (end of inspiration effort until end of pressure support; positive values indicate delayed cycling, and negative values indicate premature cycling) (Figs. 2 and 3 and Table 1), intrinsic PEEP, PEEPi (pressure at inspiration start minus external PEEP), and additional expiratory pressure-time product (PTPE) (pressure-time product above PEEP after end of inspiration effort)12,17 (Fig. 4). The chosen cycling criteria were confirmed by correlating them to the measured flow as a percentage of peak inspiratory flow at the end of pressure support (r > 0.9, R2 > 0.9).
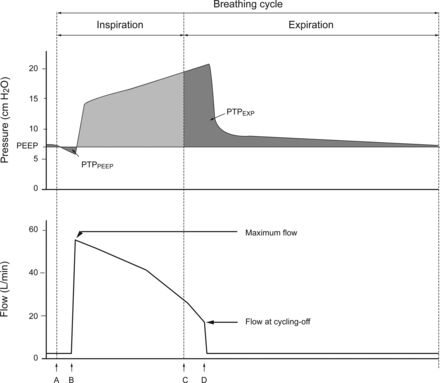
Schematic display of one breathing cycle. Shown is an example display of a pressure-time curve (Pressure ASL [cm H2O]) and a flow-time curve (Flow Ventilator [L/min]) of a pressure-supported breath cycle in ventilation via face mask. The vertical dashed lines mark the beginning (A) and the end (C) of a simulated inspiration effort. The distance A to B represents the inspiratory trigger latency; the distance C to D represents the expiratory cycle latency. PTPEXP = expiratory pressure-time product.
Whisker plot of expiratory cycle latency (TLEXP) in all tested conditions. Displayed are the values for expiratory cycle latency together for both interfaces, both respiratory rates, and both pressure support values. In low cycling criteria, trigger latency shows large values picturing delayed off-cycling. Raising the cycling criteria reduces trigger latency. The best setting in our study was 50% peak flow, taking all tested conditions into account (see also Table 2). Overdoing it by raising cycling criteria further led to negative expiratory cycle latency values describing premature cycling, the higher the off-cycling the more negative values get.
Expiratory cycle latency (TLEXP) according to height of pressure support. Both interfaces and both respiratory rates are displayed together; each ventilation situation is displayed as one mark. It can be seen that trigger latency values vary much more using high pressure support. Separated high values in both plots are cycling criteria of 10 and 20%. This figure shows clearly that low cycling criteria led to large delays, raising cycling reduced delay markedly.
Expiratory Cycle Latency With the Different Interfaces and Settings (Mean Values and SD in ms)
Compilation of Termination Criteria That Allowed the Best Synchrony (ie, Expiratory Cycle Latency Differing Least From Zero in the Given Situation; Mean and SD in ms Shown in Parentheses)
Whisker plot of expiratory pressure-time product: Direct comparison of expiratory pressure-time product (PTPEXP) in face mask versus helmet in all tested situations. A very low cycling of 10% shows a large additional expiratory pressure load. Raising cycling criteria slightly already shows a large effect; this effect of diminishing expiratory pressure load continues until 40% (helmet) or 50% (face mask), respectively, where the least additional pressure load can be seen. The lowest values correlate closely with expiratory cycle latency (see Fig. 2).
Custom-made software was used to process, analyze, and store data. For each setting, 45 breaths were recorded, and the first 5 were discarded to exclude unstable conditions. Values were produced by adding cursors to the recorded flow and pressure tracings and calculating the values as area under the curve. Statistical analysis was performed using Statistica software (Statistica 10.0, StatSoft, Tulsa, Oklahoma). Multivariate analysis of variance was performed assuming a significant difference at P < .05. As post hoc analysis, the Bonferroni adjusted t test was performed. The results were comprehensively grouped in cycling categories of low (10 and 20%), medium (30, 40, and 50%), and high (60 and 70%) cycling.
Results
Low to Medium Cycling Criteria (20 and 30%)
Switching from low to medium cycling revealed a highly significant decrease in expiratory cycle latency (P < .001), the same was true for PTPE (P < .001). Differences in PEEPi by switching from low to medium cycling were not significant using the face mask except for high f/high PS (P = .03). Using the ventilation helmet and switching from low to medium cycling showed significant reductions in PEEPi in both f/high PS (P < .001).
Medium to High Cycling Criteria (50 and 60%)
Switching from medium to high cycling also revealed a highly significant decrease in expiratory cycle latency (P < .001). In the face mask, reductions in PEEPi were only significant in high PS (P < .001). In the ventilation helmet, high f/high PS showed significance (P < .001). Reductions in VT were highly significant in the face mask (P < .001) except for high f/high PS (P = .51). In the ventilation helmet, only settings with high PS (P < .001) and high f/low PS (P < .001) showed significance.
Low Cycling Criteria (10 and 20%)
Asynchrony was found to be present in high f/high PS and mostly in the face mask. No double-triggering was observed in low cycling criteria.
In all tested situations, high values of expiratory cycle latency were observed. In the majority of measures, increasing cycling was able to reduce expiratory cycle latency significantly (P < .001) except for high f/high PS in both (face mask, P = .40; ventilation helmet, P = .96). Larger values were observed using the ventilation helmet in both f/low PS compared with the face mask, whereas in high PS, larger expiratory cycle latency was seen in the face mask.
Large PTPE values were seen in both interfaces in high PS compared with low PS; in low PS, PTPE values were moderate. Increasing cycling decreased PTPE significantly in almost all measures except for high f/high PS in both interfaces, where no difference was seen.
In both interfaces, settings of low f/low PS created negative PEEPi values. For both NIV interfaces in low f/low PS and high f/high PS, no changes in PEEPi were seen by raising cycling criteria (P = .39). All other situations showed significant influence of cycling (P < .001).
Raising cycling in the ventilation helmet showed significant changes in VT in both f and low PS (P < .001) but not in both f and high PS (P = .005 and P = .72, respectively). In the face mask, low f/low PS and high f/high PS revealed no significance (P = .71 and P = .21, respectively); all other settings showed significant influence of cycling adjustment (P < .001).
Medium Cycling Criteria (30, 40, and 50%)
No asynchrony was found in any setting without leakage; in settings with leakage, little asynchrony was found. Double-triggering was seen only with the 50% cycling criteria, low f/low PS face mask and low f/both PS ventilation helmet, respectively, in these cycling rates.
All tested situations showed a constant significant (P < .001) decrease in expiratory cycle latency throughout changes in cycling. In many settings, the value of expiratory cycle latency closest to zero was found in this cycling range.
In all tested situations, PTPE showed lower values than in low cycling criteria. In most settings, a constant decrease in PTPE was seen throughout medium cycling (P < .001); exceptions were face mask low f/low PS and ventilation helmet low f/low PS and low f/high PS, where increases appeared from 40 to 50% cycling (P < .001). When changing cycling, slight decreases and, less frequently, increases in PEEPi were seen, mostly not significant; only high f/high PS showed constant significant influence of cycling (P < .001). Generally, increasing cycling decreased VT constantly (P < .001); only ventilation helmet high f/low PS showed no significance at all.
High Cycling Criteria (60 and 70%)
No asynchrony was observed in any situation of high cycling criteria, whereas double-triggering appeared in all situations of low f but none of high f. In settings with leakage, double-triggering was seen mainly in high PS. In all settings, expiratory cycle latency values were lower than in low or medium cycling, and increasing cycling led to a significant decrease of expiratory trigger latency (P < .001).
When using the ventilation helmet, raising cycling showed a significant reduction in PTPE in all f and PS settings (P < .001). When using the face mask, reductions were seen in low f (P < .001), but in high f, increases in PTPE appeared; these were significant in high PS (P < .001). Reductions in PEEPi were significant in the face mask low f/low PS and high f/high PS and in the ventilation helmet low f/high PS (P < .001); all other settings showed no significant influence of changes in cycling.
VT reduction appeared in all settings as a result of increasing cycling. Reductions in VT were more distinct in settings with high PS compared with low PS and in low f compared with high f. All of these reductions were significant (P < .001) except for ventilation helmet high f/low PS (P = .92).
Discussion
Our results show that adjustments of cycling significantly reduce pressure load and therefore the work of breathing in a COPD simulation. Furthermore, our results show that the cycling setting must be individualized, since response time of the respiratory system, the interface, amount of pressure support, breathing frequency, and leakage will probably affect how a patient responds.14,15 Careful analysis of flow and pressure tracings and derived parameters are necessary. We recommend raising cycling criteria above the conventional level as long as parameters suggesting delayed cycling (non-supported inspirations, prolonged mechanical inspiration time, and flow curve analysis) are detectable.
Expiratory cycle latency was significantly influenced by cycling criteria. expiratory cycle latency values were more negative at high cycling criteria and low frequencies within the helmet than the mask. This finding can be explained by the interface's mechanical properties. By using it, a second elastic structure next to the lung is integrated into the breathing circuit, which influences inspiratory flow and pressure rise time.18 Initially, flow during inspiration is influenced by the mechanical characteristics of the helmet,19 whereas later, pressure transmission happens faster, and obstructive lung mechanics become predominant. In the mask, pressure transmission is much more rapid, and therefore lung mechanics gain influence much earlier.
Delayed cycling from an increase in expiratory cycle latency promotes non-supported inspirational efforts. In these situations, pressure support often continued into the next breathing cycle, causing high PEEPi and ineffective triggering. Raising cycling in our study reduced asynchrony to zero and significantly reduced PEEPi.20,21 This is consistent with previously published data that showed reduced wasted efforts with conventional set cycling (25% peak flow)22,23 compared with a higher incidence of non-supported inspiratory effort with short expiration time and high PEEPi.11,24 Shortened expiration times and expiratory flow limitation in COPD promote incomplete exhalation and PEEPi.9,25 Early cycling causes PEEPi because of negative expiratory trigger latency, which leads to double-triggering, caused by ongoing inspiration after ceased pressure support being misinterpreted as another inspiration. We observed non-supported inspiration only in high pressure support via face mask because triggering was difficult due to high PEEPi.23,24,26–28 By increasing cycling to 40%, non-supported inspiration could completely be avoided due to its effect on expiratory cycle latency.
Due to impaired flow and pressure transmission, much longer inspiration time was necessary in the helmet to trigger a ventilator breath. Therefore, double triggering was less frequent with the helmet than the mask and occurred more often with low pressure support and low frequencies because expiratory cycle latency was negative more frequently.22 PEEPi was large at high frequencies because of shortened expiration time; increasing cycling criteria causally corrected it. Adjusting cycling is therefore of critical importance, especially at high frequencies.
Leakage is known to crucially delay cycling,22,29–31 especially in obstructive lung mechanics,31 since the time to reach the pressure target and flow-based cycling criteria are prolonged. Without leakage we did not find any wasted efforts with the helmet and only very few occasions with the mask, whereas others observed increased non-supported inspirations.18,19,23,26 This difference might be explained by a difference in PEEP, which affects pressure transmission and trigger sensitivity, inspiratory force, and the performance of the ventilator.
Large PTPE values may lead to impaired exhalation, poor patient comfort, and use of accessory muscles. Ideally, PTPE should be zero. Increasing cycling led to a constant decrease of PTPE because expiratory cycle latency was reduced. This resulted in decreased pressure application during expiration. Premature cycling, on the other hand, causes double-triggering and consequently increases PTPE. PTPE could be reduced by raising cycling with both interfaces but was much lower with the helmet. This reduced expiratory pressure load is a potential advantage of this NIV interface. However, the main cause for reduced PTPE is assumed to be an already reduced PTPINSP and thus less efficient unloading during inspiration.18 We confirmed the findings of a clinical study that found a distinct expiratory pressure load in ventilation with high pressure support,12 and our study also found larger PTPE at high pressure support. Therefore, adjusting cycling should especially be considered at high pressure support.
Comparing the 2 investigated interfaces, cycling in the helmet was influenced by its compliance and volume, resulting in largely negative expiratory cycle latency compared with the mask. Until now, the use of ventilation helmets has been limited by poor synchronization, but adjusting cycling improves synchronization. This could add to patient comfort and therefore make it even more advantageous for patients who do not tolerate face masks well. Considering leakage, cycling needs to be raised to achieve a good synchronization.
There were limitations to this study. By using a well-accepted lung model27,32–34 that enables exact simulation of breathing movements, we were able to perform a systematic investigation of varying cycling criteria to a degree impossible in a clinical study. Nevertheless, a simulator only approximates the complexity of the lung itself and respiratory mechanics. Furthermore, inspiration is simulated on the theoretical basis that pressure and volume have a linear relation and compliance and resistance are constant, which is nearly true in relaxed spontaneous breathing35 but not in impaired lung mechanics and high flow velocity.10
Using a sophisticated lung model with stable setup, SD was small, which might overestimate significant differences even if negligible. Therefore, the clinical relevance of differences requires consideration.
The study was performed with a ventilator capable of adjusting cycling within a wide range. Due to varying performance, the use of another ventilator might show different results.31 A different set of NIV interfaces will certainly have an impact, since internal volume and compliance might vary,34 as lately shown for a new ventilation helmet where no significant asynchrony has been found.33,36
We kept the different settings constant irrespective of the interface used to permit comparable results.37 Especially in the helmet, the efficiency of pressure transmission and patient-ventilator interaction can be improved by compensating for its larger compliance.37 Therefore, the interface properties should be considered when applying NIV. The chosen settings might be criticized. We chose a low and a high pressure support as examples. As can be seen from our data, higher pressure support levels might worsen expiratory pressure load. A PEEP of 7 mm Hg was used as a compromise between a high and a low PEEP, based on the knowledge that the performance of the helmet decreases at a lower PEEP,18 and was kept constant to avoid adding more complexity to an already complex protocol. The effects of PEEP on cycling were not studied, and further investigations are needed here. In a recent clinical trial in COPD subjects, the mean PEEP found by the authors in the helmet group was 7.7 mm Hg as well.36
Conclusions
During noninvasive pressure support ventilation of COPD patients, inefficient setting of the cycling affects patient-ventilator interaction and might lead to increased work of breathing, tidal overinflation, and failure of NIV. Instead of relying on fixed “default” settings, cycling needs to be individualized, since synchronization is influenced by the chosen interface, frequency, height of pressure support, leakage, and ventilation mode. According to our results, best synchrony in a simulated COPD model ranged between 30 and 70% cycling. We recommend raising cycling criteria above the conventional level as long as parameters suggesting delayed cycling (non-supported inspirations, prolonged mechanical inspiration time, and flow curve analysis) are detectable.
Footnotes
- Correspondence: Onnen Moerer MD, Department of Anesthesiology, Emergency and Intensive Care Medicine, Georg-August University of Göttingen, Robert-Koch-Str. 40 D-37099 Göttingen, Germany. E-mail: omoerer{at}med.uni-goettingen.de.
Supplementary material related to this paper is available at http://www.rcjournal.com.
This study was fully financed by departmental funding. The Department of Anesthesiology, Emergency and Intensive Care Medicine, University of Göttingen provided educational courses on lung protective ventilation and hemodynamic monitoring partially supported by Maquet Critical Care and CareFusion and Pulsion Medical. Dr Quintel has disclosed a relationship with companies related to the spectrum of critical care medicine, including manufacturers (including Maquet Critical Care, CareFusion, Pulsion Medical Systems, Novalung, CytoSorbents, and Gambro). The other authors have disclosed no conflicts of interest.
See the Related Editorial on Page 122
- Copyright © 2016 by Daedalus Enterprises