Abstract
Mechanical ventilation has, since its introduction into clinical practice, undergone a major evolution from controlled ventilation to various modes of assisted ventilation. Neurally adjusted ventilatory assist (NAVA) is the newest development. The implementation of NAVA requires the introduction of a catheter to measure the electrical activity of the diaphragm (EAdi). NAVA relies, opposite to conventional assisted ventilation modes, on the EAdi to trigger the ventilator breath and to adjust the ventilatory assist to the neural drive. The amplitude of the ventilator assist is determined by the instantaneous EAdi and the NAVA level set by the clinician. The NAVA level amplifies the EAdi signal and determines instantaneous ventilator assist on a breath-to-breath basis. Experimental and clinical data suggest superior patient-ventilator synchrony with NAVA. Patient-ventilator asynchrony is present in 25% of mechanically ventilated patients in the intensive care unit and may contribute to patient discomfort, sleep fragmentation, higher use of sedation, development of delirium, ventilator-induced lung injury, prolonged mechanical ventilation, and ultimately mortality. With NAVA, the reliance on the EAdi signal, together with an intact ventilatory drive and intact breathing reflexes, allows integration of the ventilator in the neuro-ventilatory coupling on a higher level than conventional ventilation modes. The simple monitoring of the EAdi signal alone may provide the clinician with important information to guide ventilator management, especially during the weaning process. Although, until now, little evidence proves the superiority of NAVA on clinically relevant end points, it seems evident that patient populations (eg, COPD and small children) with major patient-ventilator asynchrony may benefit from this new ventilatory tool.
- neurally adjusted ventilatory assist
- NAVA
- mechanical ventilation
- patient-ventilator interaction
- synchrony
- diaphragm
Introduction
Mechanical ventilation is one of the most commonly applied techniques in the treatment of patients suffering from respiratory insufficiency in the intensive care unit (ICU). The prognosis of these patients is determined not only by the underlying condition, but also by the ventilatory strategy pursued by the clinician.1
From a physiological point of view, mechanical ventilation is indicated when there is a marked discrepancy between the level of respiratory effort required to maintain proper gas exchange and the performance capacity of the respiratory system. The main objectives of the institution of mechanical ventilation are the recovery of the gas-exchange function, and the reduction of the respiratory muscle effort. This approach allows critically ill patients to recuperate from their acute disease.
From Controlled Mechanical Ventilation to Assisted Mechanical Ventilation
Initially, a ventilator was a pump-driven machine, designed to deliver an air-oxygen mixture to the patient in a steady volume and frequency (controlled mechanical ventilation). However, those early types of ventilators did not take into account the patient's own respiratory effort. The neural respiratory cycle of a patient on ventilation is often only partially suppressed, and it is very frequently asynchronous with the delivered breathing frequency and volumes by the ventilator. In such conditions, the administration of sedation is often the tradeoff in order to improve the patient-ventilator synchrony and, ultimately, to enable adequate ventilation and gas exchange.
From the early 1980s, a new generation of ventilators has been developed able to provide assisted mechanical ventilation. These ventilators are equipped with a pneumatic sensor designed to detect the start of the patient's inspiratory effort. The pneumatic sensor mechanism detects inspiration, either by the reversal of the expiratory flow (flow sensor) or by a drop in the pressure (pressure sensor) generated by the patient's inspiratory effort. In response, the ventilator will assist the patient's inspiratory effort through a previously set inspiratory pressure assist. As the inspiratory gas flow decelerates toward the end of the inspiratory phase, to approximately 25–30% of the maximum gas flow, the expiratory valve of the machine is opened, so that the patient can exhale. This ventilation mode is known as pressure support ventilation or assisted spontaneous breathing. Assisted ventilation modes are characterized by inspiratory timing synchrony between the patient's inspiratory effort (neural inspiration) and the ventilator's delivery of inspiratory flow (mechanical inspiration).
Despite the advantage of inspiratory timing synchrony in assisted ventilation modes, recent research revealed that these modes suffer from marked patient-ventilator asynchrony. Typically, in patients with COPD a marked timing asynchrony between the expiratory phase of the breathing cycle (neural expiration) and the opening of the ventilator's expiratory valve (mechanical expiration) may be present.2,3 As discussed further in this paper, patient-ventilator asynchrony is associated with adverse outcomes.4,5
In order to resolve these problems, a new ventilation mode, proportional assist ventilation (PAV), was proposed. PAV provides assisted pressure support ventilation by means of a complex algorithm based on the compliance and resistance of the airways. PAV ameliorates timing synchrony and allows adaptation of the amplitude and the slope of the ventilatory flow assist to the ventilatory need of the patient (flow synchrony). The correct implementation of PAV implies an estimation of the airway resistance and compliance, which in clinical practice may be difficult to perform. In addition PAV is available only on a limited number of ventilators and has, until now, never been widely adopted by clinicians.
The latest development in this field, which became available to clinicians only recently, is neurally adjusted ventilatory assist (NAVA). This new mode of assisted mechanical ventilation provides proportional pressure support based on measurements of the electrical activity of the diaphragm (EAdi), which serves as a proxy for the neuronal output of the respiratory center.
The Neuro-Ventilatory Coupling
The neuro-ventilatory coupling is the complex process that coordinates the neural ventilation and the mechanical ventilation. The neural respiratory center is located in the medulla of the central nervous system. Via an oscillating network of inspiratory and expiratory neurons, it controls the breathing cycle and generates the respiratory pattern. The respiratory center is influenced and modulated by signals from other parts of the brain, including from the motor cortex, so that we are able to consciously control our breathing to some extent. All these signals are coordinated in the pons; together they ultimately determine our respiratory neuronal activity. This information is integrated and modulated by the spinal cord through reflexes originating in receptors in the lung, thorax, and respiratory muscles. The neural signal initiates, via the phrenic nerve, the muscle fiber action potential of the diaphragm, resulting in a mechanical contraction of the diaphragm. The intensity of this mechanical contraction is dependent upon the number of activated motor units, which is in turn determined by the intensity and frequency of the neural activity. The activation of the respiratory muscle results in an expansion of the thorax through the generation of a negative intrathoracic pressure, causing lung expansion and a negative alveolar pressure, generating an inspiratory air flow in spontaneous breathing. Throughout the whole breathing cycle the respiratory center is continuously affected by a complex neural feedback mechanism originating from receptors located in the brain and at the peripheral level.
The neuro-ventilatory coupling can therefore be viewed as a complex interaction resulting in initiation and modulation of the respiratory cycle. To this day we still lack a device that can measure the neural activity generated by the respiratory center and the neural feedback. The present generation of ventilators is equipped with flow, pressure, and volume sensors. However, changes in flow, pressure, and volume are merely the result of neuro-ventilatory coupling. Knowing that, in healthy individuals, the latency time between the neural activity and the initiation of inspiration is less than 20 ms, it would seem reasonable to assume that a sensor located further upstream is desirable (Fig. 1). An integration of the ventilator with a higher-level neuro-ventilatory coupling would—in theory at least—also be conducive to a much enhanced patient-ventilator synchrony.6–8
Neuro-ventilatory control sequence, from neural inspiratory signal to (mechanical) inspiration. The closer the sensor to trigger inspiration is located to the central controller, the better the technology is integrated with the neuro-ventilatory coupling. Neurally adjusted ventilatory assist (NAVA) responds to diaphragm excitation. (From Reference 6, with permission.)
Patient-Ventilator Asynchrony
The crucial points in the respiratory cycle are (1) trigger (the time point at which the ventilator is triggered and the inspiratory assist begins), (2) cycle (the time point at which the expiratory valve of the circuit is opened), and (3) the level and the speed of the pressurization during the inspiratory phase.
A complete review of patient-ventilator asynchrony is beyond the scope of this paper. Therefore we refer the reader to other excellent reviews.4,9,10 Basically, from a physiological point of view, patient-ventilator asynchrony may be divided into timing asynchrony and flow assist asynchrony.
Timing asynchrony refers to a discrepancy between the timing of the neural respiratory cycle of the patient and the respiratory cycle of the ventilator. Timing asynchrony may relate to the beginning of the inspiratory phase (Fig. 2) or the beginning of the expiratory phase (Fig. 3). Auto-triggering (initiation of a mechanical assist without inspiratory effort) and ineffective efforts (absence of mechanical assist despite inspiratory effort, see Fig. 2) both can be considered as the extremes of timing asynchrony.
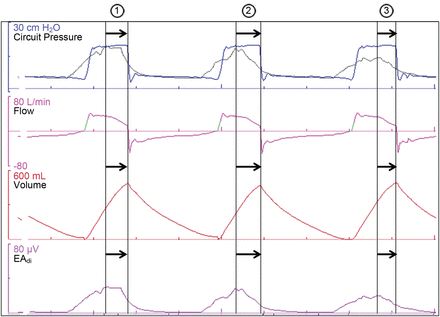
Pressure, flow, volume, and electrical activity of the diaphragm (EAdi) waveforms from a patient on pressure support ventilation, and the presumed pressure curve (grey) if the patient was on neurally adjusted ventilatory assist (NAVA), with a preset NAVA level not shown. Breaths 1 and 3 demonstrate inspiratory timing asynchrony: there is a delay between the neural inspiration and the mechanical inflation. In breath 1 there may also be assist asynchrony. The amplitude of the neural breathing effort is greater than that of the mechanical inflation. Breath 2 demonstrates ineffective triggering: despite the presence of a neural breath effort, there is no mechanical inflation. Ineffective triggering asynchrony can be considered an extreme form of timing asynchrony.
Pressure, flow, volume, and electrical activity of the diaphragm (EAdi) waveforms from a patient on pressure support ventilation, and the presumed pressure curve (grey) if the patient was on neurally adjusted ventilatory assist (NAVA), with a preset NAVA level not shown. Breath 3 demonstrates expiratory timing asynchrony: there is continued mechanical inflation after the start of the neural expiration, causing a delay between the neural expiration and the mechanical deflation.
Flow assist asynchrony refers to a discrepancy between the amplitude of the neural respiratory output and the level of inspiratory assist provided by the ventilator. Flow assist asynchrony may relate to the flow rate or the amplitude of the mechanical ventilatory assist (see Fig. 2). There is little available data on flow assist asynchrony. A “physiological” variation in the tidal volume and breathing pattern would, in any case, seem plausible.
The Importance of Patient-Ventilator Synchrony
Patient-ventilator asynchrony may occur in up to 25% of patients on assisted mechanical ventilation.3–5,11,12 Patient-ventilator asynchrony occurs primarily in patients with a high intrinsic respiratory frequency, in patients with chronic airway obstruction, and in patients who are ventilated with a high level of pressure support resulting in a tidal volume exceeding their habitual tidal volume.3,4 Patient-ventilator asynchrony can be attributed to pathological patient-related factors and to technical factors (inappropriate ventilator settings or technical defect of the ventilator).9,10
The main patient-related determinant of substantial patient-ventilator asynchrony during assisted ventilation modes is the presence of intrinsic PEEP and dynamic hyperinflation. The latter, as commonly observed in patients with COPD, leads to a shortening of the inspiratory muscles, with a reduced apposition zone of the respiratory muscle and a more horizontal position of the diaphragm. This, in turn, compromises diaphragm muscle strength, resulting in a delayed activation of the ventilator's pneumatic trigger, compared to the beginning of the neural inspiration. If, moreover, the inspiratory pressure increases too slowly, or if the pressure level is too high, then the opening of the ventilator's expiratory valve will occur after the beginning of the neural expiration, resulting in continued mechanical inflation after the beginning of neural expiration. An excessive inspiratory pressure increase will result in excessive tidal volume, triggering the Hering-Breuer reflex, and hence an additional depression of the patient's neural respiration. If the high minute volume results in respiratory alkalosis, then the respiratory reflex will be depressed even more, resulting in apneas.13
Technical factors such as inadequate setting of the pneumatic trigger threshold may also result in major patient-ventilator asynchrony. A too sensitive inspiratory trigger threshold, as well as air leaks from the circuit, may result in auto-triggering. On the contrary, a too high pneumatic trigger threshold will result in excessive delay of mechanical inflation or, in extreme situations, will result in absence of mechanical inflation.2
Patient-ventilator asynchrony is associated with (1) an increased need for sedation and neuromuscular blockade, (2) increased transpulmonary pressure, with a risk of barotrauma and ventilator-induced lung injury, and (3) prolonged duration of mechanical ventilation.3–5,11,12 In addition, enhanced patient-ventilator synchrony is associated with greater patient comfort and better quality of sleep.14 Inadequate quality of sleep is associated with a greater risk of delirium and may contribute to mortality. Prolonged ventilation is associated with a greater risk of ventilator-associated pneumonia, prolonged ICU stay, a higher incidence of tracheotomy and—last but not least—higher costs. It seems likely that this morbidity can, in itself, lead to greater mortality.5
Neurally Adjusted Ventilatory Assist
NAVA may be considered as a mode of assisted mechanical ventilation where the level of ventilatory assist is proportional to diaphragm muscle electrical activity (EAdi). The diaphragm is the principal respiratory muscle. The electrical activity generated by the diaphragm, as captured by the EAdi signal, is a measure of the patient's neural effort. In this manner, the ventilator is “connected,” as it were, to the patient's own respiratory center. The timing and intensity of the EAdi signal both determine the timing and intensity of the ventilatory assist, resulting in a high level of synchrony between the neural respiratory cycle and the flow of the ventilator. Contrary to most conventional modes of assisted mechanical ventilation that use solely a pneumatic trigger, NAVA takes advantage of the EAdi signal as an “electric” trigger.
The implementation of NAVA as an assisted ventilation mode assumes that the EAdi is a reliable measure of the patient's neural respiratory drive.15 Moreover, the patient's neuro-ventilatory coupling needs to be intact in order for the intensity of the EAdi signal (as an expression of the neuronal activity of the respiratory center) to be proportional to the patient's ventilatory requirement. This implies that all the receptors of the respiratory system's feedback mechanism are sufficiently functional and that their signals are interpreted correctly by the respiratory center. In critically ill patients, however, this feedback mechanism and the respiratory center itself may be affected and compromised in many different ways. Lung injury, central or peripheral nervous system injury, sedation and analgesia, or the mechanical ventilation mode itself may all affect this complex feedback mechanism.16,17
The Electrical Signal From the Diaphragm
The EAdi signal is the sum of the electrical activity of the diaphragm. This EAdi signal includes both the frequency and the intensity of the diaphragmatic muscular activity and is expressed in microvolts (μV). The EAdi signal is measured transesophageally by means of an EAdi catheter, which has 8 bipolar microelectrodes mounted on the tip of a gastric tube. It is positioned near the crural diaphragm, where the EAdi signal can be measured. The EAdi signal is the resultant of a complex algorithm that filters the electrical signals of the diaphragm from those emitted by the heart, the esophagus, and other surrounding muscles.18,19 The resulting signal is amplified and displayed as the EAdi curve. Hitherto, NAVA is available only on the Servo-i ventilator (Maquet Critical Care, Solna, Sweden).
Before implementing mechanical ventilation with NAVA, the respiratory practitioner must properly place the EAdi catheter to obtain an accurate EAdi signal. In practice, the correct position of the electrode is determined on the basis of several criteria20:
The anatomical reference based on the presumed distance between the crural diaphragm and the tip, and calculated with a formula
The electrocardiogram signal, which can be visualized on a specific screen on the ventilator console and which, because of the aspect of the P wave and the QRS wave, is indicative of the position of the electrode
The synchrony of the EAdi signal with the negative deflection of the airway pressure curve in an inspiratory effort against an occluded artificial airway
In principle, one should always be able to capture the EAdi signal, except in cases of major anatomical defect (eg, diaphragmatic hernia); central apnea without respiratory drive (eg, sedation, brain damage); or in the absence of electrical diaphragmatic activity (eg, phrenic nerve damage, muscle relaxants).
However, dislocation of the nasogastric tube may disrupt the EAdi signal, so that further ventilation with NAVA may be hampered or even made impossible. The ventilator is, however, equipped with a safety mechanism that switches to pressure support ventilation if no EAdi signal is detected. If, moreover, the patient is not displaying spontaneous respiratory drive, then the ventilator will, courtesy of an additional safety mechanism, switch to a pressure-controlled mechanical ventilation.
Interpretation of the Diaphragm Signal
The absolute value of the EAdi signal exhibits significant inter-individual variation. Hence, one cannot put forward a normal value for the EAdi signal. While it is not entirely clear precisely which factors come into play here, they are believed to be both anatomical and physiological in nature. As regards the anatomical aspects, it is assumed that the distance from muscle to electrode may vary from patient to patient. Additionally, the motor unit density of the diaphragm can also differ between individuals, so that the intensity of the diaphragmatic electrical activity measured may also vary.
Physiologically it is assumed that an individual recruitment of the diaphragm muscle and motor units underlies a higher or a lower EAdi signal. This motor unit recruitment is codetermined by the physiological reserve of the neuro-ventilatory coupling. In a “healthy” person at rest the EAdi signal is lower than in a chronic pulmonary patient at rest. Presumably the latter is an expression of a limited physiological reserve of the neuro-ventilatory coupling. A patient with respiratory insufficiency has an excessively high EAdi signal as an expression of an insufficient reserve of the neuro-ventilatory coupling, which fails to meet the patient's respiratory needs.3,21–23
The EAdi signal may also vary intra-individually, depending on the stage of disease and the physiologic reserve of the respiratory system.24 Thus, the evolution of the EAdi signal can be used as a measure of the progression of the respiratory function and possibly help predict the success of weaning from mechanical ventilation.
The Respiratory Cycle in Assisted Spontaneous Ventilation With NAVA
Mechanical inflation starts when the ventilator detects a deflection of the EAdi signal greater than the set threshold (mostly 0.5 μV). During the inspiratory phase of the respiratory cycle the mechanical assist is adapted to the instantaneous EAdi signal, which is measured every 16 ms and amplified by a set NAVA level. The resulting pressure assist above PEEP (in cm H2O) equals thus the NAVA level (in cm H2O/μV) multiplied by the EAdi signal (in μV). This means that the final mechanical assist delivered by the ventilator in NAVA will be proportional to the neural output as measured by the EAdi signal. As there may be breath-to-breath variation in the EAdi signal, there may also be breath-to-breath variation in tidal volume with NAVA. When the EAdi signal reaches 40–70% of the peak EAdi signal, the ventilator ceases inspiratory assist and opens the expiratory valve.
In addition to the “EAdi trigger” based upon the EAdi signal, there is still pneumatic triggering possible with NAVA. Both triggers operate in combination on a first-come-first-served basis. As a backup, if the EAdi signal disappears completely, the ventilator switches to a pressure support ventilation mode. Finally, as a second backup the ventilator can switch to pressure-controlled mechanical ventilation if spontaneous breathing efforts disappear.
In practice this means that when a patient completes a respiratory cycle on NAVA, the duration and the intensity of the ventilatory assist will correspond with the duration and intensity of the neural respiratory cycle. As patient-ventilator synchrony is adequately guaranteed throughout the respiratory cycle with NAVA, one could argue that the breathing cycle of a patient ventilated with NAVA is integrated on a higher level into the neuro-ventilatory coupling mechanism.25
The NAVA Level
The assumption that NAVA allows integration of the ventilator into the neuro-ventilatory coupling implies that the patient controls the transpulmonary pressure during the respiratory cycle through feedback to the neural respiratory activity, as measured on the basis of the EAdi signal.7 The choice of the NAVA level for a particular patient in a particular situation is based on that principle.
The respiratory therapist sets the NAVA level with an empirical titration procedure26 with stepwise increase of the ventilatory assist. During this titration procedure the patient will sequentially go through a phase of under-assist, a phase of adequate assist, and finally a phase of over-assist. It is important that the clinician recognizes these phases in order to select an adequate level of ventilatory assist.
A patient with respiratory insufficiency ventilated with a too-low NAVA level, resulting in insufficient pressure increase, will result in an under-compensation of the respiratory demand by the ventilatory assist. Clinically, the patient will express a rapid shallow breathing pattern. The neural output of the respiratory center will exhibit an excessively high intensity, resulting in high EAdi signals as a sign of respiratory distress.
If the NAVA level is progressively increased with increasing inspiratory pressure, the amplitude of the EAdi signal will progressively decrease until a plateau is reached where the tidal volume is constant. The patient is now in a “comfort zone” with a minute volume that meets his respiratory demands with maximal unloading of the respiratory muscles.
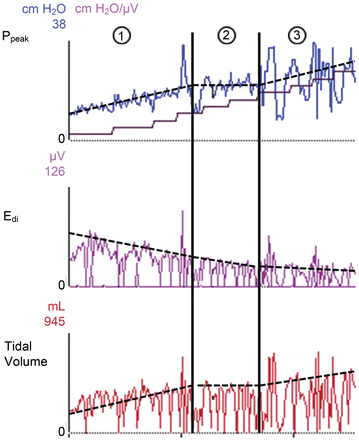
If the NAVA level is further increased, resulting in excessive pressure increase, the amplitude of the EAdi signal will decline, but in conjunction with an increase of the tidal volume. The patient is then in a zone of overcompensation. Such a high pressure level may be dangerous because of lung overdistention and the associated risk of ventilator-induced lung injury. However, even at an excessively high NAVA level the EAdi signal will never disappear entirely. Figure 4 shows an example of a NAVA titration procedure.
Peak pressure (Ppeak), electrical-activity-of-the-diaphragm (EAdi), and tidal volume waveforms from a patient on neurally adjusted ventilatory assist (NAVA), with stepwise increasing NAVA level. In the top graph the stair-step curve shows the stepwise increasing NAVA level. With increasing NAVA level the Ppeak and tidal volume increases while EAdi decreases. In column 1 the patient suffers from insufficient assist: with intact neuro-ventilatory coupling, the patient increases the respiratory efforts by augmenting the EAdi signal in an attempt to augment the tidal volumes. In column 2 the EAdi signal continues to decrease slowly while Ppeak and tidal volume remain constant: this is the so-called “comfort-zone” where the patient is adequately unloaded. If the tidal volume meets the patient's demand, the patient will decrease his effort with increasing NAVA level.27 In column 3 the EAdi signal continues to decrease while Ppeak and tidal volume increase: in this part of the curve the ventilator assist is considered inadequately high.
In the so-called “comfort zone” the tidal volume is determined by the patient himself. In this zone, the patient “chooses” a tidal volume and a breathing frequency that meets his respiratory demand. In other words, the patient largely determines the arterial CO2 tension. Experience has shown that patients who are ventilated with NAVA are able to maintain an acceptable PaCO2 and tidal volume with a more variable breathing pattern, as compared with pressure support ventilation.8,27
Potential Benefits and Applications of NAVA
The clinical experience with NAVA in humans is small. Based on the sound physiological principles, however, many potential applications exist.
Titration of the Pressure Support Level
The monitoring of the EAdi signal allows titration of the pressure support level in assisted mechanical ventilation more adequately to the needs of the patient.27 More in particular, the EAdi signal could help to recognize situations where the pressure support is set too high.
An excessively high pressure support setting leads to a prolonged mechanical inspiration, continuing beyond the duration of the neural inspiration phase and into the neural expiration phase. In patients whose Hering-Breuer reflex is intact, the neural expiration phase will also be extended, resulting in a reduced respiratory frequency. Traditionally, a low respiratory frequency in patients on pressure support ventilation is seen as an indication of “improvement.” However, if the pressure support is set too high, this may in fact be an expression of a neural inhibition of the respiratory function induced by patient-ventilator asynchrony.28 The reason is that the patient is actually receiving excessive pressure support, and the clinician gets the impression that the pressure support level cannot be reduced and that the patient is not weanable. This kind of assist asynchrony can be diagnosed only by means of EAdi signal monitoring.
Moreover, an excessive pressure support level can induce an apnea, whereby the EAdi signal remains “flat.” In combination with auto-triggering caused by an excessively sensitive pneumatic trigger, this may mislead the clinician as, in this situation, the respiratory rate is no longer representative of the diaphragmatic activity. This situation can also only be recognized through monitoring of the EAdi signal.29
Titration of the PEEP Level and Tonic Diaphragm Activity
In addition, monitoring of the EAdi signal, particularly in young children, can also be useful in recognizing tonic diaphragm activity and improving the PEEP setting. It has been documented that mainly young children (< 1 year) demonstrate not only pronounced patient-ventilator asynchrony, but also tonic diaphragm activity that persists during the expiratory phase of the respiratory cycle. The tonic EAdi is considered a mechanism to protect from lung derecruitment by maintaining the end-expiratory lung volume in acute lung injury. This tonic EAdi has a negative impact on the phasic EAdi. Titration of the PEEP level in accordance with the EAdi signal could optimize the respiratory pattern and reduce tonic diaphragm activity, allowing the patient to maintain spontaneous ventilation in NAVA.30,31 Observational data from small populations suggest that NAVA in these patients is feasible and seems well tolerated.32,33
NAVA in Noninvasive Ventilation
The use of a pneumatic cycle-on criterion in noninvasive ventilation is prone to air leakage, which is an important problem, commonly occurring around the face mask. Such leakage may induce patient-ventilator asynchrony and possibly failure of the noninvasive ventilation. The EAdi signal is not affected by air leakage and could therefore be used as an electrical cycle-on criterion, theoretically ensuring better patient-ventilator synchrony. In healthy volunteers, noninvasive ventilation with NAVA seemed to improve patient comfort, which may improve patient tolerance.34,35
Monitoring of the Diaphragm Signal During Mechanical Ventilation and Weaning
Arguably the most important purpose of EAdi signal monitoring is the diagnosis of the diaphragmatic activity itself. The EAdi signal could help determine the appropriate sedation depth and mechanical ventilation strategy. EAdi-based sedation might reduce the use of deep sedation and shorten the duration of mechanical ventilation. As prolonged diaphragmatic inactivity might contribute to the development of diaphragmatic atrophy, the qualitative monitoring of the diaphragmatic activity with the EAdi signal might be helpful in the prevention of ventilator-induced diaphragm dysfunction.36 The quantitative monitoring of the EAdi signal, such as the evolution of the EAdi signal and the response of the EAdi signal to the imposed ventilator load, could be used as an additional weaning parameter. Weaning trials could explore whether a critical increase in the EAdi signal may serve as a predictive factor for weaning failure. On the other hand, a “normalization” of the EAdi signal might be predictive of successful weaning and extubation. The potential benefit of the EAdi signal as a new monitoring parameter remains, however, speculative until clinical trials prove otherwise.
Lung-Protective Mechanical Ventilation
Animal experiments suggest that NAVA is lung-protective, compared to low-tidal-volume ventilation in early acute lung injury. With NAVA the animals were allowed to choose their own breathing pattern, resulting in preserved diaphragmatic contraction and better cardiac performance. From a physiological point of view, one may speculate that spontaneous ventilation with enhanced patient-ventilator synchrony through NAVA may reduce the risk of ventilator-induced lung injury.36
Indications for NAVA
It is hard to determine clear indications for mechanical ventilation with NAVA. Most studies thus far have been physiological studies conducted on animals and healthy persons. Probably, patients with major patient-ventilator asynchrony are most likely to benefit from NAVA. This group might include small children and COPD patients, who frequently exhibit intrinsic PEEP and dynamic hyperinflation. However, all mechanically ventilated patients able to sustain spontaneous assisted ventilation with an intact neural ventilatory drive, and who have no contraindications for nasogastric intubation, are eligible. Obviously, large clinical trials in the ICU are needed to clarify this issue.
Conclusions
From a physiological point of view, NAVA is a new exciting tool that has been recently introduced in clinical practice. Its unique feature is to rely on EAdi to trigger the respiratory cycle and to deliver proportional assist in harmony with the patient's neural drive on a breath-to-breath basis. NAVA offers better patient-ventilator synchrony than other available modes of assisted ventilation. Although there is, until now, no direct evidence from human clinical trials that better patient-ventilator synchrony with NAVA results in better outcomes, it remains a very promising tool both for clinicians and researchers working in the field of mechanical ventilation. On the other hand, both improvements in EAdi catheter cost and availability on multiple ventilators are needed before widespread application of this mode in the future seems possible.
Acknowledgments
We thank Hilde Fleurackers and Kim De Rycke for secretarial assistance.
Footnotes
- Correspondence: Walter Verbrugghe MD, Department of Critical Care Medicine, Antwerp University Hospital, Wilrijkstraat 10, B 2650 Edegem, Belgium. E-mail walter.verbrugghe{at}uza.be.
-
The authors have disclosed no conflicts of interest.
- Copyright © 2011 by Daedalus Enterprises Inc.