Abstract
BACKGROUND: The efficacy of symmetrical-waveform high-frequency oscillating (HFO) air flow for airway secretion clearance is controversial and debated in the literature.
METHODS: We conducted in vitro experiments with ovine tracheae to investigate the effects of symmetrical-waveform HFO on tracheal transport of artificial mucus. We mounted each trachea as an intact tube, with a 15o head-down tilt, infused artificial mucus (10 mL over one hour) at the caudal end of the trachea, and measured mucus-transport velocity as the time between the beginning of infusion and the first appearance of artificial mucus over 2 near-infrared sensors at the rostral end of the trachea and by measuring the amount of mucus emerging. In a second series of experiments we opened each trachea flat and with video microscopy we measured the transport velocity of plaques over the endogenous mucus sheet.
RESULTS: In the intact-trachea preparation, HFO at 20 Hz and 50 cm H2O increased mucus-transport velocity from 5.8 mm/min to 7.8 mm/min. HFO led to nearly half the artificial mucus being cleared during the infusion period. In the opened-trachea experiments the mean control transport velocity was 8.7 mm/min, and HFO, at 14 Hz or 20 Hz (and 50 cm H2O), did not significantly alter that velocity.
CONCLUSIONS: Symmetrical-waveform HFO increases mucus-transport velocity and mucus clearance when a thick layer of mucus is present. This may be important when considering the mechanisms of mucus clearance and using HFO for secretion clearance.
Introduction
The ability of high-frequency oscillating (HFO) air flow administered to the airway lumen to modify lung mechanics and alveolar gas exchange has been established and is used in clinical practice.1 As early as 1985 it was reported that orally applied asymmetrical-waveform HFO increased mucus clearance,2 but the effectiveness of symmetrical-waveform HFO, which can be achieved with many conventional HFO generators, has not been objectively confirmed, although many airway-clearance devices without biased flow are widely used in clinical practice.3 One possible explanation for this lack of evidence is that early studies suggested that an asymmetric oscillation pattern (with the highest flow during the expiratory phase) is required to maximize mucus clearance.4–7 The lack of an appropriate model for objective comparison of different methods, devices, and protocols has led to inconclusive clinical trials. A recent Cochrane review considered 265 studies and concluded that “there was no clear evidence that oscillation was a more or less effective intervention overall than other forms of physiotherapy.”8
Many methods have been used to measure non-cough mucus movement, including measuring the mass of the mucus as it appears at the output end of the system, and mucus-transport velocity, as an index of mucociliary efficiency.9–15 Nonetheless, mucus-transport velocity does not indicate the total mass of mucus moved. To measure both mucus-transport velocity and mucus mass transport we developed a novel ovine trachea model designed to reproduce as closely as possible the situation in the in vivo trachea. We used this model to investigate the effects of symmetrical-waveform HFO on mucus transport.
The hypothesis tested was that symmetrical-waveform HFO would facilitate the clearance of excessive mucus, as occurs in cystic fibrosis16 or COPD.17 We used a commercially available polyethylene oxide powder to create an artificial mucus with mechanical properties similar to endogenous mucus,4,18,19 although the artificial mucus lacks some of the moieties in natural mucus. In a separate experiment we assessed the effect of symmetrical-waveform HFO on basal mucus transport.
Methods
The study was conducted at Massey University, Palmerston North, New Zealand. Dr Tatkov was partly supported by a joint grant from the New Zealand Foundation of Research, Science, and Technology and Fisher & Paykel Healthcare. Fisher & Paykel Healthcare had no role in the study design, data collection, interpretation, writing, or editing of this paper.
From a local abattoir we obtained tracheae of farm-reared lambs of 20–30 kg. The tracheae were transported to the laboratory in Krebs-Henseleit solution, at ambient temperature, and mounted as preparations within 60 min. The Krebs-Henseleit solution was equilibrated (gassed) with carbogen prior to use, but was not gassed further during the mucus-collection period. Each trachea was either mounted as a complete tracheal tube, to measure the transport rate of infused artificial mucus, or cut open and mounted flat, to measure mucociliary transport of endogenous mucus.
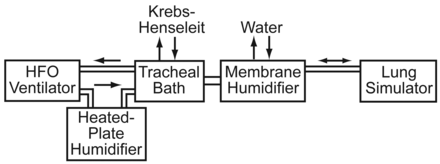
In both experiments a reciprocal air flow was generated over the epithelium with a computerized breathing simulator (ASL 5000, IngMar Medical, Pittsburgh, Pennsylvania) connected to the trachea's caudal end, to mimic a typical breathing pattern and airway resistance. This was basically a one-compartment lung model. We used a tidal volume of 0.35 L, a respiratory rate of 25 breaths/min, and a simulated distal airway resistance of 5 cm H2O/L/s. We attached a membrane-type humidifier (FC-125–240-5PP, Perma Pure, Toms River, New Jersey) between the lung simulator and the preparation, to ensure the air from the simulator was at 38°C and fully saturated with water vapor. Once the trachea was mounted, its adventitial surface was bathed in recirculating, gassed, Krebs-Henseleit solution at 38°C (flow 50 mL/min, reservoir 2 L).
We connected an HFO ventilator (SLE2000HFO, SLE, South Croydon, United Kingdom) to the trachea's laryngeal end, and applied continuous positive airway pressure of 5 cm H2O and a continuous symmetrical-waveform HFO of 14 Hz or 20 Hz, at an amplitude of 50 cm H2O. The air from the ventilator was warmed to 38°C and fully saturated by a heated-plate respiratory humidifier (MR730, Fisher & Paykel Healthcare, New Zealand) (Fig. 1). Temperature and humidity were monitored at both ends of the trachea, using an established technique.20 The humidifiers were adjusted to ensure that temperature and humidity remained within 0.1% of 38°C and relative humidity > 98%, respectively. Once the control experimental conditions were established, each trachea was allowed to stabilize for 20 min.
Setup for both experiments.
Tube Trachea Preparation
The intact sheep tracheae (length 160 mm) were mounted in a custom-made tissue bath, such that both ends of the trachea were sealed, but there was no obstruction to mucus flow between the 2 ends. The trachea was placed with a 15° cephalad-end-down tilt, to mimic postural drainage, as in in vivo experiments.6
We attached a dual near-infrared light scattering probe to the outside of the cephalad end of the trachea. The probe had 2 reflective near-infrared sensors (HOA2498-002, Honeywell), which we placed in the rostro-caudal line, and a near-infrared long-pass optical filter (Thermoset ADC filter N43-952, Edmund Optics, Singapore). The output from the paired emitters and detectors was synchronously detected at 1 kHz, to reduce interference from asymmetrical extraneous light, and amplified. The signal passed through a 10-Hz low-pass filter, purpose-built conditioning amplifier, and was captured with an analog-to-digital converter (PowerLab 4ST, ADInstruments, Sydney, Australia).
We created an artificial mucus of 1.6% polyethylene oxide powder (Polyox [molecular weight 5,000,000], Union Carbide, Danbury, Connecticut) in phosphate-buffered saline, and infused the artificial mucus at the caudal end of the trachea with a micro-infusion syringe pump (Mi60-1CZ, World Precision Instruments, Sarasota, Florida) at 10 mL/h. The artificial mucus was collected at the cephalad end in pre-weighed containers, and weighed (accuracy ± 1 mg). We infused 10 mL of artificial mucus over 60 min, and we collected the transported artificial mucus from the rostral end every 10 min during the infusion period and for 30 min after the infusion was stopped.
In the experimental group of 10 tracheae we applied HFO at 20 Hz and 50 cm H2O, from the beginning of the mucus infusion to the end of the experiment (90 min). The control group of 10 tracheae received the same mucus infusion without HFO. The mucus-transport velocity was calculated from the time between the start of the infusion at the caudal end of the trachea and its detection by the proximal sensor of the near-infrared probe at the cephalad end.
Flat-Mounted Trachea Preparation
The construction of the tissue bath used for the flat-mounted preparations has been described previously.9 We cut a sheep trachea lengthwise along the ventral midline, opened it, and, with minimal stretching, mounted it with pins over the bathing fluid container. It was held in place with a poly-methyl-methacrylate top that fit over the pins. The preparation was left for at least one hour prior to the experiment to allow dissipation of any mediators released by the mechanical trauma of mounting. The mounting was set up on a vibration-proof table. We viewed the epithelium with a modified dissecting stereo microscope (OMPI 1FC, Carl Zeiss, Germany) with a digital video camera (DCR-TRV33E, Sony, Japan) attached to one eyepiece. We calculated the mucus-transport velocity based on measurement of the movement of plaques in the mucus across a graticule. We digitized images of plaques that stayed on the surface of the mucociliary lining within the focal plane of the camera long enough for measurements to be made. This preparation had the advantage that it allowed mucus movement to be timed over a few seconds, which allows detection of rapid changes, which is not possible with the tube model, in which mucus movement was timed over the whole length of the preparation.
We tested 24 trachea preparations in the bath system: 8 controls, 8 with HFO at 20 Hz and 50 cm H2O for 30 min, and 8 with HFO at 14 Hz and 50 cm H2O for 30 min. Each experiment was of 90 min duration, with HFO generated from the 30th to 60th minute. One-minute video recordings were made every 15 min. Two additional videos were made 1 min after the oscillator was switched on or off, to look for immediate, short-term effects.
The mucus-transport-velocity data from both the artificial and endogenous mucus experiments were normally distributed, and we used the unpaired 2-tailed Student t test and 2-way independent analysis of variance accordingly. Some groups in the data on artificial mucus output did not pass the normality test, so we analyzed those data with the non-parametric Mann-Whitney test.
Initially, we planned to perform 10 experiments with each preparation, but after power analysis at the end of the 8th experiment with the flat preparation we decided to discontinue this series, as the observations were not going to be significant if the full protocol was completed.
Unless otherwise stated, the data are expressed as mean ± SD and effect size (r). We report median and effect size (r) for the values from the non-parametric Mann-Whitney tests. Differences were considered statistically significant when 2-tailed P < .05.
Results
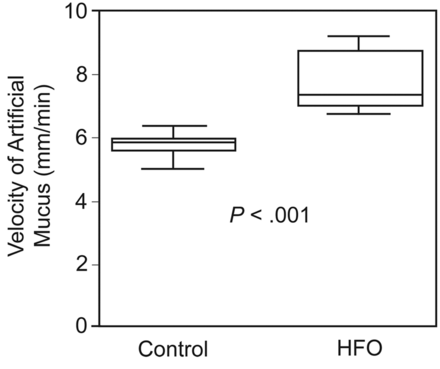
In the control preparations the mean mucus-transport velocity was 5.8 ± 0.3 mm/min. This was significantly increased by HFO (Fig. 2) (P < .001, r = 0.88); however, both means were within the range of previous observations in our laboratory. The increased mucus-transport velocity was reflected in the rate of mucus output. In both groups, mucus first appeared in the third sample collection period, at 30 min after the infusion was started. In the later collection periods the rate of mucus accumulation in the HFO preparations exceeded that of the controls (Table 1). In the last collection period during the mucus-infusion period (ie, 50–60 min), this difference decreased to nonsignificant (1.17 ± 0.14 g vs 1.08 ± 0.25 g), largely due to an increase in output in the control preparations. During the 30-min collection period after infusion was stopped, the mucus output in the HFO preparations declined, whereas that in the control group increased (Fig. 3), probably because in the HFO preparations almost half of the infused mucus (median 4.77 mL per preparation) had cleared during the infusion period, compared to 2.86 mL per preparation in the control preparations (P < .001, r = 0.85). In the post-infusion period significantly more mucus was collected in the control group (median 3.82 mL vs median 2.80 mL, P = .001, r = −0.78). In the HFO group, mucus output during the infusion period was significantly higher. When the infusion was stopped, significantly more mucus was collected in the control group (see Table 1).
Mucus-transport velocity with the control setup and the high-frequency oscillation (HFO) setup. In each box, the horizontal line indicates the median. The top and bottom of the box indicate the 25th and 75th percentiles, respectively. The whiskers show the minimum and maximum values.
Effect of High-Frequency Oscillation on Transport of Artificial Mucus*
Artificial mucus transported to the cephalad end of the intact tube trachea preparation. The artificial mucus (10 mL) was infused during the first hour. In the high-frequency oscillation (HFO) preparations nearly half of the mucus was cleared during the first hour. In the control group the mucus accumulation rate increased more slowly, causing more mucus to be delivered to the cephalad end of the trachea after the infusion period (ie, in minutes 60–90). In each box, the horizontal line indicates the median, the top and bottom of the box indicate the 25th and 75th percentiles, respectively, and the whiskers show the minimum and maximum values.
Figure 4 summarizes the data from the second series of experiments. In the control group the mean of all mucus-transport velocity measurements was 8.7 ± 3.2 mm/min. Repeated-measured analysis of variance indicated that HFO had no effect on mucus-transport velocity (F(2, 168) = 0.602, difference not significant), that there was no significant effect of time (F(7, 168) = 0.449, difference not significant), and that there were no significant interactions between groups and time (F(14, 168) = 0.024, difference not significant).
Effect of high-frequency oscillation (HFO) on mucus-transport velocity, measured by timing the movement of mucus plaques across a video image of the exposed epithelial surface. HFO, at 14 Hz or 20 Hz, was delivered for 30 min. Neither HFO frequency significantly affected endogenous mucus transport. In each box, the horizontal line indicates the median, the top and bottom of the box indicate the 25th and 75th percentiles, respectively, and the whiskers show the minimum and maximum values.
Discussion
Our first experiment investigated whether symmetrical-waveform HFO assists tracheal mucus clearance, with a mucus volume similar to that in patients with severe COPD17 or cystic fibrosis.16 We obtained a crude measure of overall mucus transport by collecting the mucus output at the rostral end of the preparation and measuring its mass, which is a method previously employed by Freitag et al.6 We determined mucus-transport velocity by measuring the time between the start of mucus infusion and its first appearance at the sensors at the rostral end of the trachea. It is interesting that the mucus movement peaked during the 40–50-min and 50–60-min collection periods in the HFO preparations, an in the 70–80-min period in the control preparations. This indicates that HFO enhanced the mucus-clearance rate.
We also investigated HFO's influence on basal mucociliary transport, using a flat preparation of ovine trachea.9 This preparation had several advantages over using the trachea as a tube. Reducing the distance over which the mucus is timed allows measurement of mucus-transport velocity over a few seconds. Also, investigating the rate of transport over different parts of the image allowed us to establish that the rate was evenly distributed.
With bovine trachea, Wills et al found that clearance was dependent on the mechanical properties of the mucus they obtained from patients with various respiratory disorders.21 Wills et al also found that some of the responses of mammalian airway were contrary to those of amphibian palate,22 which has been the classical model for mucociliary transport.23,24 Taken together, the above studies indicate that the in vitro mammalian trachea may be a sensitive model with which to assess the effects of exogenous factors on mucociliary function.
In most of our experiments we used a frequency of 20 Hz and a pressure swing of 50 cm H2O, both of which were the maximum the ventilator would generate and, hence, its maximum high-frequency power output. Our rationale was based on the disparate reports in the literature on the effects of symmetrical-waveform HFO. We wanted to see if there was any effect in the preparations, rather than try to quantify the effects under different conditions. We also tested HFO at 14 Hz, the justification for which was that Freitag et al7 found that it was the most efficient frequency for HFO, and King et al observed that 13 Hz (very close to 14 Hz) delivered to the airway lumen had no significant benefit, but instead appeared to inhibit mucus transport.25
By using a reciprocal air flow to simulate tidal breathing and conditioning the air flowing in both directions, the intact tube preparation essentially embodied the situation that probably occurs over most of the length of the trachea in vivo. This makes the tube preparation a more robust model than monodirectional air flow. Warming and 100% humidification of the gas was important, as a previous study in our laboratory showed that even minor humidity perturbations can compromise ovine mucociliary transport.9
Possible Mechanisms for the Increased Mucus Transport Velocity
In previous studies artificial mucus was infused at the very high rate of 1 mL/min,6,19 presumably to generate the lining thickness of 5–10% of the airway diameter, which experiments with glass tubes had shown to be required if the 2-phase gas/liquid flow mechanism was to have a major effect.19 But it is unlikely that mucus flow of 1 mL/min could continue long-term in a patient, even with the most extreme pathological conditions. Rather than inject large volumes of artificial mucus, to permit the 2-phase gas/liquid flow mechanism to have a role, we injected it at 10 mL/h, which is more in keeping with sputum production during treatment in a hypersecreting patient with severe COPD.17 Since we infused artificial mucus at 10 mL/h, and it was 30 min before a substantial amount appeared at the laryngeal end of the preparation, it follows that during the experiment the trachea contained up to a maximum of 5 mL of artificial mucus. If we assume that the mucus is evenly distributed around the airway, we can estimate the increase in thickness of the lining fluid. While this assumption is almost certainly invalid, it indicates that the contribution of the infusate to the mucus thickness would be hardly enough to allow the 2-phase mechanism to play an important role in the studies discussed here.
Two other mechanisms appear possible. Either the oscillations altered the rate or efficiency of ciliary action, or the mucus rheology was modified. It is possible that superimposing HFO on tidal breathing increased the shear force on the airway mucosa, which may have induced mediator release (possibly adenosine triphosphate [ATP]) and, hence, increased mucus transport by cilia. ATP is released in response to air-flow-induced shear stress, which up-regulates ion transport and increases mucociliary transport.26 ATP also stimulates the release of glycoprotein from submucosal glands.27 However, in our second experiment we found no changes in mucus-transport velocity, which indicates that this probably did not happen in our experiments.
It is well known that vibration reduces the viscosity of synthetic polymers.28 Experiments with magnetic ball rheometers (a classic method of studying respiratory mucus29) indicated that this is also the case for respiratory mucus. The transport velocity of mucus-simulant gel was directly related to mucus elasticity and the depth of the periciliary fluid, and inversely related to mucus viscosity.30
Both endogenous and artificial mucus exhibit shear thinning, and high shear thinning forces produced by HFO may at least temporarily decrease viscosity, which would make mucus flow more readily. However, that effect is probably transient and restricted to the period of HFO.
Limitations
Our use of polyethylene oxide powder artificial mucus to stimulate the volume of mucus produced in some respiratory diseases was a necessary compromise. In each of the main experiments we infused 10 mL over one hour, so the whole series required 200 mL of a mucoid substance, and collecting that volume of endogenous ovine mucus was impractical. Whereas the polyethylene oxide powder we used had the closest physical properties of all the commercially available polymers that have been tested,4,18,31 it lacks some of the other moieties in tracheal liquid lining that influence mucociliary transport. While a single polymer can be used to mimic the mechanical effects of an increased mucus layer, it does not reflect the complex chemical makeup of natural mucus. Particularly important is surfactant,32,33 which probably has multiple roles. It may be involved in the partitioning of inhaled particles between the sol and gel phases of the lining liquid34,35 or act as a wetting agent.36 However, by far its most important effect is its capacity to increase mucus-transport velocity in both animal models37 and patients.31,33,38
Endogenous airway lining fluid also contains other moieties that may influence mucociliary transport, including ions and molecules that may be mediators that alter mucociliary transport and DNA. The presence of DNA in the mucus of patients with cystic fibrosis is well established, and DNA's molecular size is thought to alter mucus mechanical properties and, hence, clearance.39
Conclusions
Symmetrical-waveform HFO increased the mucus-transport velocity and clearance of relatively large volumes of an infused mucus-like substance from ovine tracheae mounted with a 15o head-down tilt. HFO's improvement of airway clearance is dependent on excess mucus being present; HFO does not affect clearance in an airway with a typical thickness of endogenous mucus under standard physiological conditions. Our in vitro model with reciprocal flow of conditioned air can be used for objective evaluation of various intraluminal airway-clearance methods. This objectivity is difficult to achieve in the clinical setting.
Footnotes
- Correspondence: Rodger J Pack PhD, Institute of Food, Nutrition, and Human Health, College of Sciences, Massey University, Private Bag 11 222, Palmerston North, New Zealand. E-mail: r.j.pack{at}massey.ac.nz.
-
This study was partly supported by a joint grant from the New Zealand Foundation of Research, Science, and Technology, and Fisher & Paykel Healthcare.
- Copyright © 2011 by Daedalus Enterprises Inc.