Abstract
BACKGROUND: When polysomnography is indicated in a patient with a presumed sleep disorder, continuous monitoring of arterial carbon dioxide tension (PaCO2) is desirable, especially if nocturnal hypoventilation is suspected. Transcutaneous CO2 monitors (PtcCO2) provide a noninvasive correlate of PaCO2, but their accuracy and stability over extended monitoring have been considered inadequate for the diagnosis of hypoventilation. We examined the stability and accuracy of PtcCO2 measurements and the performance of a previously described linear interpolation technique designed to correct for calibration drift.
METHODS: We compared the PtcCO2 values from 2 TINA TCM-3 monitors to PaCO2 values from arterial blood samples obtained at the beginning, every 15 min of the first hour, and then hourly over 8 hours of monitoring in 6 hemodynamically stable, male, intensive care patients (mean age 46 ± 17 y).
RESULTS: Time had a significant (P = .002) linear effect on the PtcCO2-PaCO2 difference, suggesting calibration drift over the monitoring period. We found no differences between monitor type or interaction between time and monitor type. For the 2 monitors the uncorrected bias was 3.6 mm Hg and the limits of agreement were −5.1 to 12.3 mm Hg. Our linear interpolation algorithm improved the bias and limits of agreement to 0.4 and −5.5 to 6.4 mm Hg, respectively.
CONCLUSIONS: Following stabilization and correction for both offset and drift, PtcCO2 tracks PaCO2 with minimal residual bias over 8 hours of monitoring. Should future research confirm these findings, then interpolated PtcCO2 may have an increased role in detecting sleep hypoventilation and assessing the efficacy of treatment.
Introduction
When polysomnography is indicated in a patient with a presumed sleep disorder, continuous monitoring of arterial carbon dioxide tension (PaCO2) is desirable, especially if nocturnal hypoventilation is suspected. Arterial blood sampling provides direct assessment of blood gas tensions, but it is an invasive, expensive, potentially sleep-disruptive measure, and provides only periodic data. In contrast, transcutaneous monitoring of PCO2 (PtcCO2) provides continuous data and is noninvasive. Transcutaneous CO2 monitors employ a skin-surface Stow-Severinghaus electrode to measure CO2 after heating and “arterialization” of the underlying tissue.
Although continuous PaCO2 measurement is desirable in the monitoring and treatment of hypoventilation, transcutaneous CO2 monitoring is specifically not recommended for the quantification of hypoventilation during a sleep study.1 The limits of agreement between PaCO2 and PtcCO2 are considered too imprecise, particularly for extended monitoring where there is increased potential for electrode calibration drift. The methods used to quantify the change in PtcCO2-PaCO2 difference (electrode drift) during sleep are not well established, and previous research has not clearly established whether electrode drift is systematic and therefore amenable to quantification and correction. Despite these limitations, both the absolute PtcCO2 value and the PtcCO2 change during sleep are frequently reported as a clinical measure of both disease severity and treatment efficacy, particularly in studies of the outcome of noninvasive ventilation.2–7 Although the minimum clinically important change that PtcCO2 monitoring aims to measure is not known, current consensus guidelines suggest that a rise of ≥ 10 mm Hg in PaCO2 indicates sleep hypoventilation,1 and recent publications have suggested that 7.6 mm Hg should be the accepted minimum clinically important difference between measured PaCO2 and PtcCO2.8,9
To better understand the relationships between PtcCO2 and PaCO2, we assessed the stability and accuracy of PtcCO2 readings from 2 TINA TCM-3 monitors (Radiometer Medical, Brønshøj, Denmark) during extended monitoring, and determined the limits of agreement between PtcCO2 and PaCO2 measured from arterial blood, to characterize the monitors' electrode drift over 8 hours. In our institution the accuracy of the PtcCO2 values during a sleep study is established with an in vivo calibration against arterial blood samples taken at the start of the monitoring period. Any observed PtcCO2-PaCO2 difference thereafter is assumed to behave as a fixed offset and to remain constant. A second arterial sample is taken at the end of the monitoring period, and the observed change in the PtcCO2-PaCO2 difference is assumed to reflect electrode drift. To assess the validity of this procedure we also assessed the utility of the previously published methods for correction of offset and electrode drift in PtcCO2 values.10
Methods
This research was conducted at Austin Hospital, Heidelberg, Australia. The study protocol was approved by the Human Ethics Committee of the Austin and Repatriation Medical Centre. All patients or their authorized representatives gave informed consent.
Subjects
It was considered unethical to conduct arterial puncture in otherwise healthy people undergoing a routine sleep study, so we recruited stable patients in our intensive care unit (ICU) to serve as a model for people in the sleep laboratory. We selected ICU patients because they typically have an indwelling arterial catheter. The potential impact of tissue hypoperfusion on the results was minimized by excluding patients on inotropic support, with temperature greater than 37.0°C, or who just had cardiac arrest or surgery.
Transcutaneous Carbon Dioxide Monitor
We measured PtcCO2 simultaneously with 2 TINA TCM-3 monitors (Monitor 1 and Monitor 2). The TCM-3 monitor employs a dual transcutaneous carbon dioxide and oxygen electrode. We used a new transcutaneous electrode membrane for each study. The electrodes were re-membraned 12–24 hours prior to each study. The probes were heated to 43°C and the analog outputs recorded continuously on chart paper and were calibrated with the devices' 1-volt internal test signal.
The PtcCO2 monitors were calibrated according to the manufacturer's instructions, using a one-point dry-gas calibration with 5% CO2. The skin was shaved if required, cleansed with alcohol, and dried. The PtcCO2 electrodes were positioned approximately 2 cm apart on the patient's anterior chest wall, and attached with their respective adhesive rings and contact solutions. Additional tape was applied to secure the electrodes and minimize the risk of movement or dislodgment. The usual nursing, medical, and physiotherapy care of the patients was not altered during the monitoring period.
Arterial Blood Sampling
Arterial blood samples were obtained via a 3-way tap connected to the arterial line. Arterial samples were taken at the beginning, every 15 min for the first hour, and then hourly during the monitoring period. The clinical care of participants was managed by the usual ICU care team, but attention was given to ensure that the participants had no change in ventilation made immediately prior to any sample being drawn. Furthermore, all samples were drawn during periods of steady-state breathing and recording (defined as no PtcCO2 change > 2 mm Hg for at least 3 min and no apparent respiratory instability associated with rolling over or with gross body movements). We recorded the time of each arterial sample and the PtcCO2 value at that time of the arterial sample.
The accuracy of the blood-gas analyzer (ABL500, Radiometer Medical, Brønshøj, Denmark) was confirmed with standard quality-control solutions. Whole-blood tonometry was also performed twice during each study period to verify the accuracy of the blood-gas analyzer. The arterial blood samples were immediately placed in an ice-and-water slurry, and analyzed (in duplicate) within 20 min of drawing. If the 2 PaCO2 values were more than 1 mm Hg different, a third PaCO2 measurement was made. We used the average of the 2 closest PaCO2 values.
Method for Correcting Electrode Drift and Offset
For each patient we matched the PaCO2 values obtained 15 min after electrode application with the corresponding PtcCO2 and used the difference as the initial measurement of electrode offset. The PaCO2 measurements at 8 hours were used as the final in vivo calibration points for the simultaneous PtcCO2 measurement. We used changes in that difference as an estimate of electrode calibration drift. We corrected the PtcCO2 values by subtracting the baseline offset alone, and then by subtracting both the baseline offset and the drift over the 8 hours.10 A spreadsheet example of the correction process is available in the supplementary materials at http://www.rcjournal.com.
Statistical Analysis
Given the potential for variable or systematic PtcCO2-PaCO2 differences, we employed several strategies to analyze PtcCO2 offset and drift. We analyzed for offset and drift with linear regression of each subject's PtcCO2 recording. We analyzed the PtcCO2-PaCO2 differences and patterns of PtcCO2 change (drift) in the group data with repeated-measures analysis of variance, incorporating polynomial trend analysis to investigate systematic linear versus higher-order polynomial effects over time. We made post hoc pair-wise comparisons of mean differences at each time, incorporating a Bonferroni correction for multiple comparisons. We examined the benefit of using corrected values with a linear mixed-effects model analysis,11 with the PtcCO2-PaCO2 difference as the response, subject as a random effect with separate intercept, and monitor (Monitor 1 or Monitor 2) and correction type (uncorrected, corrected for baseline only, and corrected for both baseline and drift) as factors. We conducted the analyses with statistics software (PASW Statistics 18.0, SPSS, Chicago, Illinois), and we ran the fully saturated model first, followed by removal of nonsignificant interaction terms. We also determined instrument bias (average) and limits of agreement (1.96 standard deviations) in the group data, according to the methods of Bland and Altman.12 Results are presented as mean ± SD unless otherwise indicated. P < .05 was regarded as significant.
Results
We recruited 6 male patients (mean age 46 ± 17 y, range 26–72 y), whose diagnoses were trauma, post-neurosurgery, C3/4 spinal injury, C4/5 spinal injury, subarachnoid hemorrhage, and asthma. Four subjects were intubated and sedated to various degrees, but none were pharmacologically paralyzed. The mean PaCO2 of all 72 arterial samples was 45.6 ± 12.8 mm Hg (range 31.9–77.7 mm Hg). All samples were drawn at the scheduled sample times, and all the recording periods were 8 hours. We found no cutaneous irritation or burning associated with the electrodes.
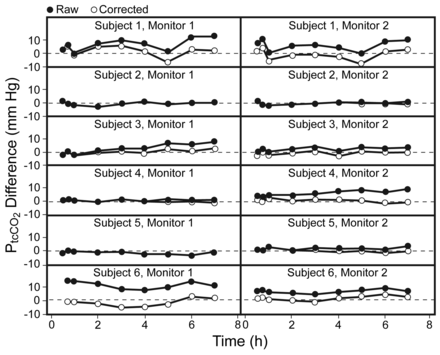
Figure 1 shows the PaCO2 and PtcCO2 values, and Figure 2 shows the PtcCO2-PaCO2 differences. Table 1 shows the minimum and maximum PaCO2, drift, and intercept data, and the linear regression correlation coefficients. In the individual recordings, statistically significant baseline offset and linear drift were common: each affected 5 of 12 records (see Table 1), and 4 of 6 patients had drift of > 4 mm Hg in at least one monitor. In the group data there was a significant positive intercept (P = .01) and linear effect of time on the PtcCO2-PaCO2 difference (P = .002), indicating systematic bias and drift, increasing on average from 1.7 ± 1.6 mm Hg at baseline to 5.4 ± 3.5 mm Hg at the conclusion of the recording period. No higher-order polynomial effects were observed, which supports the finding that the drift was predominantly linear. There was no difference in overall performance between the 2 monitors in raw PtcCO2-PaCO2 difference scores (P = .82) and no significant interaction between time and monitor type (P = .97).
PaCO2 and transcutaneously measured PCO2 (PtcCO2) measured with 2 PtcCO2 monitors, over 8 hours in 6 subjects.
Raw and corrected differences between PaCO2 and transcutaneously measured PCO2 (PtcCO2) measured with 2 PtcCO2 monitors, over 8 hours in 6 subjects.
PaCO2, Drift, Intercept, and Slope Data
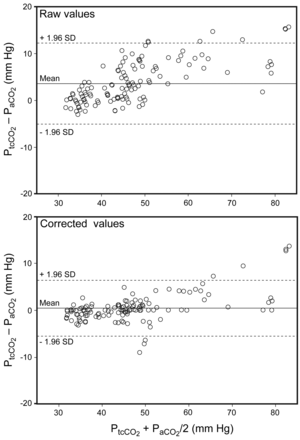
Offset and drift correction decreased the bias and limits of agreement of the PtcCO2-PaCO2 difference (see Figs. 2 and 3). Mixed-model analysis showed statistically significant main effects of time (P < .001), monitor (P = .01), and correction type (P < .001), and a significant monitor-by-correction interaction (P = .01), but no further interaction effects. The monitor main and interaction effects indicated that, overall, Monitor 1 had greater bias than Monitor 2 (2.4 ± 1.5 mm Hg vs 1.6 ± 1.5 mm Hg), P = .01), in post hoc tests, revealing greater residual bias in Monitor 1 than in Monitor 2 with a baseline correction alone, but no further differences between the monitors. The correction-type effect showed incremental reductions in PtcCO2-PaCO2 difference with each correction, and that the combined correction was superior. Overall bias was reduced by 1.7 ± 1.1 mm Hg with baseline correction alone (P = .001), and by a further 1.5 ± 0.9 mm Hg with combined baseline and drift correction (P < .001, Table 2).
Bland-Altman plot of raw and corrected (interpolated) PaCO2 and transcutaneously measured PCO2 (PtcCO2) (combined PtcCO2 values from the 2 monitors). Before correction the bias is 4 mm Hg and the limits of agreement are –9 to 9 mm Hg. After correction the bias is less than 1 mm Hg, and the limits of agreement are –6 to 6 mm Hg.
Effect of Offset and Drift Correction on the Bias and Limits of Agreement* of Transcutaneously Measured PCO2
Discussion
We have demonstrated that while PtcCO2 monitoring can track trends in PaCO2 during an extended (8-hour) monitoring period, significant electrode drift is common, but tends to be linear when PtcCO2 is stable (ie, no change > 2 mm Hg over the preceding 3 min). Consequently, a 2-point in vivo calibration using the PtcCO2 offset and drift, versus arterial PaCO2 measurements at the beginning and end of recording, allowed for substantial compensation for PtcCO2 drift. Simple baseline adjustment alone reduced bias, but ignores significant drift, and was inferior to 2-point baseline and drift correction. While the requirement for arterial blood measurements at the beginning and end of extended recording may limit the clinical utility of this method, more frequent arterial sampling is often impractical, particularly in a sleep laboratory setting, where PtcCO2 monitoring is common and potentially most useful.
We confirm that the accuracy and the agreement of the monitors was too imprecise for uncorrected PtcCO2 measurements to substitute directly for PaCO2 in adults, as has been previously described.4,13–18 The reasons for the observed differences between PaCO2 and PtcCO2 have previously been reviewed.19 However, we would attest that the aim of continuous CO2 measurement during extended sleep monitoring is to detect sleep-related changes that suggest the presence or adequate control of hypoventilation, rather than as a direct measure of PaCO2 per se (ie, monitoring changes during sleep, rather than measuring absolute values), so noninvasive devices that can detect change over an extended monitoring period with sufficient accuracy and minimal artifactual drift have substantial clinical utility.
Limitations
The generalizability of our findings is limited by our use of only one model of PtcCO2 monitor and the small number and type of patients studied. However, the performance of the TINA-TCM3 monitors was similar to that in other studies that used the TINA-TCM3 monitor2,4 suggesting that our results are valid for this device. Although we studied only 6 participants, the PaCO2 values ranged widely, from 31.9 mm Hg to 77.6 mm Hg, and that wide range has been suggested as desirable in studies such as this.4 As shown in Figure 3 and in previous literature,13,15,20 the correlation between PaCO2 and PtcCO2 decreases as PaCO2 increases, particularly beyond 50 mm Hg, which reinforces the need for both offset and drift correction to maximize the utility of a continuous PtcCO2 trace, and it cautions against PtcCO2 as the sole method of quantifying severe hypoventilation.1 Our study therefore both demonstrates the utility of the offset and drift correction method and identifies circumstances where its performance may be compromised.
We used ICU patients as “models” of patients in a sleep laboratory, because we could not conduct arterial punctures in otherwise well sleep patients. Additionally, our experience suggested that taking arterial blood samples from sleeping participants typically resulted in arousal from sleep, large changes in ventilation, and, thus, non-steady-state comparisons of transcutaneous and arterial measurements. While all care was taken to include participants who best modeled the “sleep patient,” future validation studies of the interpolation method with multiple simultaneous measurements over an extended monitoring period should be with sleeping patients who experience a wide range of PaCO2.
Similar to transcutaneous carbon dioxide monitoring, end-tidal carbon dioxide (PETCO2) monitoring is a noninvasive method of continuously measuring carbon dioxide over an extended period. In healthy awake individuals, PETCO2 is essentially equivalent to alveolar (and thus arterial) carbon dioxide values. However, data obtained during sleep indicated that PETCO2 is insufficiently accurate for diagnostic use in the able-bodied because of the normal reduction in tidal volume and the associated difficulty in detecting an alveolar plateau in the PETCO2 trace.20 Furthermore, patients with underlying lung disease and associated ventilation-perfusion inhomogeneity are likely to have additional dissociations between PETCO2 and alveolar CO2. The American Academy of Sleep Medicine guidelines1 provide a recommendation level of D for PETCO2 monitoring, and C for PtcCO2, and recommend that further research examine the ability of PtcCO2 to monitor change over extended periods. Those guidelines make no recommendation for further investment in PETCO2 monitoring during sleep. Unfortunately, there is currently no ideal noninvasive measure of CO2 during sleep; in the present study we sought to explore whether the imperfect transcutaneous measurement can be made better through the addition of arterial blood sampling at the start and end of the recording period, and by offset and drift correction
While the detection of a PaCO2 difference of 7.6 mm Hg has been suggested to be the clinically important threshold value,8,9 that value is arbitrary and may not represent a clinically relevant limit. There appear to be no published, prospective data that relate a given change in PaCO2 (much less PtcCO2) to clinical decision making or to clinical outcomes. However, Rodriguez et al demonstrated good discriminant ability of a PtcCO2 monitor to detect a 7.6 mm Hg threshold,18 and the American Academy of Sleep Medicine recommendations suggest that an overnight PaCO2 change of ≥ 10 mm Hg indicates sleep hypoventilation.1 Therefore, although 7.6 mm Hg may be arbitrary, it is a pragmatic limit that may be expected to be sensitive to clinically important change. However, longitudinal studies of a 7.6 mm Hg change are required to confirm the effect of that difference on clinical decision making or patient outcomes.
Conclusions
Even though PtcCO2 has been considered inadequate for the diagnosis or the measurement of response to treatment of hypoventilation, it is used clinically and frequently reported as a measurement of both disease severity and treatment efficacy, particularly in studies of noninvasive ventilation.2–7 This apparent contradiction suggests that clinicians use the PtcCO2 signal, most likely in combination with other polysomnographic traces and periodic PaCO2 measurements, in the care of their patients, while acknowledging that it may not accurately represent PaCO2 at all times.
The present study provides the first prospective evidence that if corrections are implemented for both offset and drift, PtcCO2 tracks PaCO2 with minimal residual bias over 8 hours of monitoring. Further research, particularly in subjects with less stable ventilation during sleep, is required before PtcCO2 is likely to be recommended for routine clinical use. However, should further research support that recommendation, the present results suggest that the bias and limits of agreement of the interpolated CO2 values are likely to be sufficiently precise to both detect sleep hypoventilation and to assess the efficacy of treatment.
Footnotes
- Correspondence: David J Berlowitz PhD, Institute for Breathing and Sleep, Bowen Centre, Austin Hospital, PO Box 5555, Heidelberg, Victoria, 3084, Australia. E-mail: david.berlowitz{at}austin.org.au.
Supplementary material related to this paper is available at http://www.rcjournal.com.
-
This research was partly supported by a Dora Lush Scholarship from the National Health and Medical Research Council of Australia, and a grant from the Physiotherapy Research Foundation of Australia.
-
The authors have disclosed no conflicts of interest.
- Copyright © 2011 by Daedalus Enterprises Inc.