Abstract
BACKGROUND: There is paucity of data from India on the use of noninvasive ventilation (NIV) in acute respiratory failure (ARF). In this observational study, we report the indications and outcomes of patients requiring NIV in the respiratory ICU of a tertiary care hospital.
METHODS: All patients with ARF requiring NIV were included in the study. NIV was delivered through critical care ventilators, using oronasal mask. The disease severity and new-onset organ dysfunction/failure were calculated using the Acute Physiology and Chronic Health Evaluation (APACHE II) and Sequential Organ Failure Assessment (SOFA) scores, respectively. A multivariate logistic regression model was used to analyze the factors predicting NIV failure.
RESULTS: There were 92 subjects (48 men, 44 women, mean ± SD age 48 ± 17.5 y) who received 101 NIV applications (42 and 59 applications for episodes of hypoxemic and hypercapnic ARF, respectively) during the study period. The most common causes of hypoxemic and hypercapnic respiratory failure were acute lung injury/ARDS (29%) and COPD (29%), respectively. There was significant improvement in heart rate and respiratory rate after 1, 2, and 4 hours, compared to the baseline, in both the groups. Of the NIV applications, 53.5% required endotracheal intubation, with the number being significantly higher in hypoxemic (67%), compared to hypercapnic (44%), ARF (P = .03). The PaO2/FIO2 measured after 1 hour of NIV application had significant impact on outcome in patients with hypoxemic but not hypercapnic ARF. A PaO2/FIO2 of ≤ 146 mm Hg at one hour had a better specificity (85.7% vs 71.4%), versus a PaO2/FIO2 of ≤ 175 mm Hg in predicting NIV failure in patients with hypoxemic ARF. On multivariate logistic regression analysis, baseline APACHE II score, ΔSOFA score, hypoxemic respiratory failure, and change in PaO2/FIO2 at 1 hour from baseline were associated with NIV failure.
CONCLUSIONS: NIV was found to be a useful modality in management of patients with hypercapnic versus hypoxemic respiratory failure. The severity of illness at admission, new-onset organ dysfunction, hypoxemic ARF, and delay in improvement in PaO2/FIO2 at 1 hour from baseline are independent predictors of NIV failure.
Introduction
Noninvasive ventilation (NIV) refers to delivery of mechanical ventilation to the lungs, using techniques that do not require an endotracheal airway. The widespread use of NIV began with the discovery that CPAP could improve gas exchange and daytime somnolence of obstructive sleep apnea.1 Subsequently, NIV has been successfully applied to chronic respiratory failure due to neuromuscular causes and severe kyphoscoliosis.2 NIV has also been evaluated in diverse causes of acute respiratory failure (ARF), and can potentially obviate the need for endotracheal intubation and reduce the occurrence of nosocomial infections, duration of ICU stay, and the overall cost of hospitalization.3
NIV is currently considered the standard of care in exacerbations of COPD, with its use associated with reduced tracheal intubation, duration of hospitalization, and mortality.4–8 On the other hand, the use of NIV in severe acute asthma is not widely accepted.9 The role of NIV in weaning and post-extubation respiratory failure also remains controversial.10,11 The application of NIV in hypoxemic ARF has also been an area of research over the last 2 decades.12 Two recent meta-analyses of randomized controlled trials found no robust evidence to support the role of NIV in hypoxemic ARF and acute lung injury (ALI)/ARDS respectively.13,14 However, the use of NIV in specific settings of hypoxemic ARF (pneumonia in immunocompromised hosts and post lung resection surgery) has been shown to reduce endotracheal intubation rates and even mortality.15–17 In ARF due to cardiogenic pulmonary edema (CPE), the use of NIV has been shown to reduce mortality in meta-analyses,18,19 but a recent large trial failed to demonstrate any survival advantage.20
We have earlier described the factors predicting outcome in patients with ARF in COPD versus other causes.21 Subsequently, we described the outcomes of patients with hypoxemic ARF in our respiratory ICU (RICU).22 Recently, we described the role of NIV in severe acute asthma.23 However, these studies from our ICU may not be a true reflection of the actual ICU practice, and are limited by a selection bias in inclusion of a specific group of patients. Moreover, in most of the ICUs in teaching hospitals in India, only the most seriously ill patients are admitted, due to overcrowding in the emergency department. There are also differences in resources, and the case mix from developing countries is likely to be different from the developed world. Hence, the outcomes with NIV application may be different. There is also great importance in international comparison of NIV data. However, there is a paucity of data on the use of NIV in ARF from India. In fact, there are only 5 published studies that have investigated the use of NIV in patients with ARF from India.21–25 In this observational study we report the indications and outcomes of all patients admitted in our RICU requiring NIV at admission or during the ICU stay.
QUICK LOOK
Current knowledge
Noninvasive ventilation (NIV) is a standard of care for exacerbations of COPD and acute cardiogenic pulmonary edema. Despite this fact, NIV remains underutilized in the United States. The utilization of NIV in India is not well known.
What this paper contributes to our knowledge
NIV was found to be a useful modality in hypercapnic respiratory failure, while its use in hypoxemic respiratory failure was less successful. Disease severity at admission, occurrence of new organ dysfunction, hypoxemic respiratory failure, and delay in improvement in PaO2/FIO2 at one hour from baseline were independent predictors of poor outcome with NIV. These findings in a tertiary care hospital in India are similar to those in North America and Europe.
Methods
Study Design and Patient Selection
This was a prospective observational study conducted in the RICU of this institution. All patients admitted to RICU between January 1, 2009, and March 31, 2010, who received NIV for management of ARF were included in this study. The RICU is an 8-bed ICU with a total of 8 pulmonary fellows (5 posted in a day), 5 consultants, and a nurse to patient ratio of 2:1. The unit has been using NIV since the year 2000, and has considerable expertise with the application of NIV. The entire faculty and all fellows are well trained during their residency in intubation and invasive ventilation. The study was approved by the institute's ethics committee, and written informed consent was obtained from all patients or the next of kin.
This study observed patients in our ICU receiving NIV based on the following established protocol. NIV is administered in patients with ARF, defined by the presence of both of the following criteria:
Clinical symptoms and signs of acute respiratory distress, such as dyspnea, respiratory rate > 30 breaths/min, use of accessory muscles of respiration, or the presence of paradoxical breathing, and
Arterial blood gas analysis showing pH ≤ 7.35 with PaO2/FIO2 < 300 mm Hg (or PaO2 < 60 mm Hg) while the patient was breathing oxygen through an air-entrainment mask.
Patients are excluded from NIV application if they have any one of the following criteria: cardiac or respiratory arrest, hypotension (systolic blood pressure < 90 mm Hg), severe encephalopathy (Glasgow coma scale score < 8), upper gastrointestinal bleeding, hemodynamic instability, unstable cardiac arrhythmia, upper-airway obstruction, inability to protect the airway and clear respiratory secretions, or abnormalities that preclude proper fit of the interface (agitated or uncooperative patient, facial trauma or burns, facial surgery, or facial anatomical abnormality).
Definitions
Pneumonia was defined by presence of fever, leukocytosis, purulent secretions, new or progressive chest radiographic infiltrates or pathological bacteria in the tracheobronchial secretions. ALI/ARDS was defined by acute onset of symptoms, bilateral pulmonary infiltrates, PaO2/FIO2 < 300 mm Hg on room air, and no clinical evidence of cardiac cause for the pulmonary infiltrates. Exacerbation of COPD was defined according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines, characterized by a change in baseline dyspnea, cough, and/or sputum beyond day-to-day variability sufficient to warrant a change in management along with respiratory acidosis, and pH < 7.35.26 Patients received NIV for post-extubation respiratory failure in the first 48 hours after extubation if they developed a respiratory rate > 35 breaths/min (or an increase in respiratory rate of > 50% from baseline) with use of accessory muscles of respiration or abdominal paradox.
Preemptive NIV for post-extubation respiratory failure was administered to those deemed at high risk of developing respiratory failure following extubation.10 High-risk patients included patients with generally 2 or more of the following factors: age > 65 years, more than one consecutive failure of weaning trial, chronic heart failure, PaCO2 > 50 mm Hg after extubation, more than one medical/surgical comorbid illness, poor cough reflex, upper-airway stridor at extubation that does not require immediate reintubation, and Acute Physiology and Chronic Health Evaluation (APACHE II) score > 12 on the day of extubation. Severe acute asthma was defined by the presence of most of the following criteria: history of asthma of at least one year; patient judged by the attending physician as having an acute attack of asthma (acute respiratory distress with wheeze, inability to complete one sentence in one breath); respiratory rate > 30 breaths/min; heart rate > 100 beats/min; and SpO2 < 92% (or PaO2 < 60 mm Hg).23 Cardiogenic pulmonary edema was defined as acute respiratory distress with respiratory rate > 30 breaths/min and orthopnea with chest radiograph findings consistent with pulmonary edema.20
NIV Protocol
NIV was delivered through the following critical care ventilators: Galileo Gold (Hamilton Medical, Bonaduz, Switzerland), Evita 2 Dura (Dräger Medical, Lübeck, Germany), and Servo-i (Maquet Critical Care, Solna, Sweden). The interface used was silicon oronasal mask with inflatable cushion (VBM Medizintechnik, Germany). NIV was started at an inspiratory positive airway pressure (IPAP)/expiratory positive airway pressure (EPAP) of 6–8/3–4 cm of H2O and was gradually increased by 2/1 cm of H2O till clinical response, in the form of relief of dyspnea, respiratory rate ≤ 30 breaths/min, tidal volume ≥ 6–8 mL/kg, and SpO2 ≥ 92% was achieved, or a maximum IPAP/EPAP of 20/10 cm of H2O was reached. During the initial 24 hours, disconnection of NIV was allowed only for intake of food and to clear oral secretions. Thereafter, depending upon the clinical response, the period off NIV was gradually increased, till patient could maintain SpO2 ≥ 92% on room air or respiratory rate of ≤ 30 breaths/min.
End Points
The demographic details of the patients, including age, sex, type of respiratory failure, indication for NIV, and the presence of comorbid illnesses were recorded. The disease severity was calculated using the APACHE II score and Sequential Organ Failure Assessment (SOFA) score. New-onset organ dysfunction/failure was computed using the change in the SOFA score (ΔSOFA), by subtracting the admission SOFA score from the maximum SOFA score during RICU stay.27 We collected the data for heart rate, respiratory rate, systolic blood pressure, diastolic blood pressure, arterial blood gases (pH, PaO2, PaCO2) at baseline, 1 hour, 2 hours, 4 hours, and every 24 hours till patient discharge. The primary outcomes were failure of NIV (defined as number of patients requiring endotracheal intubation), time to endotracheal intubation after starting NIV, and RICU and hospital stay. The secondary outcomes were improvement in clinical and blood gas values assessed at 1, 2, and 4 hours, hospital mortality, duration of NIV, maximum IPAP, and maximum EPAP, and time to maximum IPAP and maximum EPAP. Complications of NIV, such as claustrophobia, intolerance, abdominal distention, pressure sores, and nasal bridge trauma, were also noted. Patient discomfort was assessed by visual analog scale, with a scale of 0–100 marked by the ICU physician. It was marked as zero if there was no discomfort and 100 for maximum discomfort.
The decision to terminate NIV and move to invasive ventilation was based on the following criteria: failure in improvement of clinical parameters and gas exchange at 1 hour; development of alteration in sensorium; hemodynamic instability; and inability to tolerate oronasal mask. However, the final decision was left to the intensivist's clinical judgment.
Statistical Analysis
Results are presented in a descriptive fashion as number and percentage or mean ± SD unless otherwise stated. The difference between means of continuous and categorical variables was analyzed using the Mann-Whitney U and chi-square test, respectively. Improvements in clinical (respiratory and heart rate) and arterial blood gas parameters (pH, PaO2) were analyzed using multifactorial repeated measures analysis of variance, with Bonferroni adjustment for multiple comparisons; the within-groups factor was time (baseline, 1, 2, and 4 hours), and the between-groups factor was the type of ARF (hypoxemic vs hypercapnic). A survival curve was constructed to study the time to intubation in patients failing NIV, using Kaplan-Meier analysis. Multivariate logistic regression analysis was performed to derive adjusted odds ratios and 95% confidence intervals to analyze the factors predicting NIV failure. Statistical significance was assumed at a P value of < .05.
Results
Of the 313 admissions in the RICU during the study period, 185 were mechanically ventilated, 77 required only oxygen, and 51 received NIV on admission. Overall, 92 subjects received 101 NIV applications during the study period, with 42 and 59 for episodes of hypoxemic and hypercapnic ARF, respectively (Table 1). The most common causes of hypoxemic and hypercapnic respiratory failure were ALI/ARDS and COPD, respectively (see Table 1). There were 48 (52.2%) men and 44 (47.8%) women, with a mean ± SD age of 48 ± 17.5 years. The baseline characteristics of the subjects with hypoxemic and hypercapnic respiratory failure are shown in Table 2. The severity of illness assessed by APACHE II and SOFA scores were similar in the 2 groups. However, subjects with hypercapnic respiratory failure were older and there was a significant difference in baseline heart rate, respiratory rate, pH, and PaO2/FIO2 between the 2 groups (see Table 2). All subjects received appropriate medical management in addition to the NIV.
Etiology of Acute Respiratory Failure Requiring 101 Applications of NIV in the Respiratory ICU
Baseline Characteristics of 92 Subjects Who Received NIV in the Respiratory ICU
There was significant improvement in heart rate and respiratory rate after 1, 2, and 4 hours, compared to the baseline, in both the groups, and both the values significantly decreased in patients with hypoxemic, compared to hypercapnic, respiratory failure (Table 3). There was no significant change in pH values in the first 4 hours after NIV application. The PaO2 values were significantly different at 2 and 4 hours, compared to baseline, within the groups, but there was no significant difference between the groups (see Table 3). The mean ± SD IPAP and EPAP used were 12.6 ± 4.3 and 5.2 ± 1.2 cm H2O, respectively, and were not significantly different in the 2 groups. NIV was well tolerated, and patient discomfort on NIV, as assessed by visual analog scale, ranged from 0 to 100, with the median (and IQR) score being 15 (2.5–20). The most common complication observed was occurrence of pressure sore due to NIV mask. Other complications were claustrophobia, abdominal distention, and asynchrony.
Serial Clinical and Arterial Blood Gas Parameters During the ICU Course of the 2 Groups Receiving 101 NIV Applications
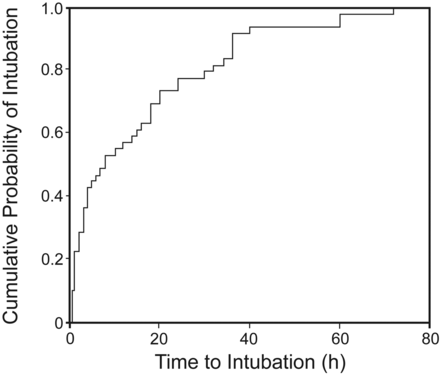
Of the 101 NIV applications, 54 (53.5%) instances required endotracheal intubation, with the number being significantly higher in hypoxemic, compared to hypercapnic, ARF (Table 4). Figure 1 depicts the time to endotracheal intubation. The mean time to intubation was 15.4 h (95% CI 10.5–20.3 h). The PaO2/FIO2 at 1 hour had significant impact on outcome in subjects with hypoxemic respiratory failure. A PaO2/FIO2≤ 146 mm Hg (or ≤ 175 mm Hg) was associated with higher incidence of failure, while those with PaO2/FIO2 > 146 mm Hg had successful outcomes in 60% of the instances (Table 5). The sensitivity and specificity of PaO2/FIO2 ≤ 146 mm Hg and ≤ 175 mm Hg in predicting NIV failure were 66.7% and 85.7%, and 75% and 71.4%, respectively. PaO2/FIO2 had no bearing on the outcome of subjects with hypercapnic ARF. The times to achieve maximum pressures with NIV, duration of NIV, time to intubation, ICU and hospital stay, and hospital mortality were similar in the 2 groups (Table 6). Out of 92 subjects, 62 (68.4%) were discharged, while 30 subjects died during the hospital stay. On multivariate logistic regression analysis, baseline APACHE II score, ΔSOFA score, hypoxemic respiratory failure, and change in PaO2/FIO2 at 1 hour from baseline were associated with NIV failure (Table 7).
Outcome of NIV in Terms of Requirement for Endotracheal Intubation Among the Various Groups of Acute Respiratory Failure
Kaplan-Meier curve of the probability of endotracheal intubation over time in the 54 subjects failing noninvasive ventilation. Most intubations occurred in the first 24 hours.
Impact of PaO2/FIO2 Scores at 1 Hour and Outcome in Subjects Receiving NIV*
Outcome Parameters During the ICU Course of the 2 Groups Receiving 101 NIV Applications
Factors Predicting NIV Failure: Multivariate Logistic Regression Model
Discussion
The results of this study suggests that NIV is not a commonly used modality in our RICU, with only 17% of patients receiving NIV at admission, and overall 30% receiving NIV at any point during their ICU stay. NIV was used most often in subjects with hypercapnic respiratory failure, with COPD constituting 28.8% of the cases, followed by other causes. The most definite indication for NIV is exacerbation of COPD,7 and COPD is generally the most common indication for NIV in many studies in patients with ARF.28–30 The most common indication in the group of patients with hypoxemic ARF was ALI/ARDS and pneumonia, similar to reported experience in the literature.15,31,32 Several studies have shown benefit in CPE;20,33 however, the numbers of subjects with CPE were few in our study, as most of the patients are admitted and managed in a separate cardiac ICU in our institute.
The success rate of NIV in this study is comparable to rates described elsewhere, both from India24,25 and other centers.34–36 NIV has been shown to be beneficial in patients with ARF of diverse etiologies.25,28,37–39 In a study, Wysocki et al, after excluding patients with COPD and hypercapnic respiratory failure, found that NIV benefitted patients with hypercapnic respiratory failure of different etiologies; however, no benefit was observed in patients of ARF without hypercapnia.40 In this study the experience was similar, with the success rate being significantly higher in hypercapnic respiratory failure, and almost two thirds of subjects with hypoxemic respiratory failure failing NIV. The failure rates of NIV in exacerbations of COPD have ranged from 5% to 40%.4,41–43 The failure rates are toward the higher side in our study, possibly due to delay in admission to the RICU because of limited ICU beds and overcrowding in the emergency department.
The use of NIV has been shown to increase the tidal volume and decrease the inspiratory muscle effort, with consequent improvement in dyspnea and oxygenation status in patients with ALI.44 Despite these physiological benefits, evidence does not support its routine use in hypoxemic ARF.13,14,22,45 In a recent meta-analysis of 13 observational studies (540 patients) investigating the use of NIV in ALI/ARDS, the intubation rates varied from 30–86%.45 This fact was also reflected in this study, with an almost 70% failure rate of NIV in pneumonia and ALI/ARDS. For similar degrees of hypoxemia, the outcome of NIV depends on the etiology of the hypoxemic ARF.46 In a study that recruited patients with pneumonia and CPE with similar degrees of hypoxemia, the outcomes in pneumonia were worse, compared to CPE, despite an initial analogous improvement in oxygenation of the 2 groups.47 A French audit also confirmed benefit of NIV only in CPE and COPD exacerbations, and not in de novo etiologies of ARF.48 Although NIV has been demonstrated to be as effective as conventional ventilation in correcting gas exchange in hypoxemic ARF,32 the presence of organ dysfunction and severity of illness are more important determinants of outcome with NIV.49–51
Currently there is no role for NIV in patients with post-extubation respiratory failure, except as a preemptive therapy in high-risk patients (for reintubation) following extubation.10 All subjects with post-extubation respiratory failure in our study required intubation following NIV, whereas NIV was successful in high-risk subjects when given preemptively to prevent post-extubation respiratory failure. Significant improvement in lung function, reduction in the dose of inhaled bronchodilator, and shorter ICU stay in subjects with severe acute asthma were observed in 2 randomized controlled trials, and a trial of NIV is justified in patients who fail standard medical therapy.23,52 In the present study all asthmatics with hypoxemic respiratory failure had successful outcomes with NIV, while hypercapnic ARF in asthma was associated with NIV failure in two thirds. NIV has been used in acute deterioration of chronic respiratory insufficiency due to various neuromuscular diseases.53 Studies have shown good success rates with NIV in myasthenic crisis,54–56 with presence of hypercapnia associated with NIV failure.54,56 In the present study NIV was given to 3 subjects with myasthenic crisis, and was successful in 2 subjects.
In many patients, after application of NIV there is inability to obtain adequate ventilation, and eventually endotracheal intubation is required for the management of ARF. The intubation rates range from 15–40% and 35–50% in COPD and non-COPD related respiratory failure, respectively.5,6,35,36,46,51 Some patients will initially benefit from NIV (for hours to a few days) but will then deteriorate and require intubation.57 The failure rates of NIV range from 5–50%, depending on the etiology and severity of the ARF.46 Delays in endotracheal intubation have been shown to be associated with decreased survival in specific patient populations (pneumocystis pneumonia in the non-HIV population or in the emergency room) being managed with NIV.46,58 In a study, Wood et al reported 43.8% failure and increase in hospital mortality in the NIV group, probably related to delay in intubation.59 Thus, it is important to ascertain the factors associated with NIV failure, so that the high-risk subset of patients likely to fail an NIV trial can be predefined. Apart from the etiology of ARF, changes in arterial blood gases have been considered the best predictors, although respiratory rate has also been found to be a good predictor of response to NIV.60,61
We found that PaO2/FIO2 at 1 hour predicted outcome in subjects with hypoxemic ARF, and a PaO2/FIO2 of ≤ 146 mm Hg or ≤ 175 mm Hg was associated with higher chances of NIV failure. Antonelli et al showed that Simplified Acute Physiology Score II ≥ 35, ALI/ARDS or pneumonia as etiology of ARF, PaO2/FIO2 ≤ 146 mm Hg after 1 hour of NIV predicted NIV failure.35 In another large study involving patients with ALI/ARDS, Simplified Acute Physiology Score II ≥ 35 and PaO2/FIO2 ≤ 175 mm Hg after 1 hour of NIV predicted a higher likelihood of intubation.51 In our study a PaO2/FIO2 of ≤ 146 mm Hg had a better specificity, as compared to a PaO2/FIO2 ≤ 175 mm Hg, meaning that failure to achieve a PaO2/FIO2 > 146 mm Hg at 1 hour is associated with higher risk of NIV failure. However, the PaO2/FIO2 at 1 hour did not predict NIV failure in hypercapnic respiratory failure. Another important predictor of NIV failure has been the severity of the underlying illness, as assessed by APACHE II or similar scoring systems,35,36 although some studies have failed to demonstrate this observation.21,22,39,62–65 In this study the baseline APACHE II scores and the ΔSOFA score predicted NIV failure. Hence, it is important not only to select patients properly, as unselected patients (eg, ALI/ARDS with shock) have uniformly poor outcomes,50 but the duration of NIV trial also requires close observation with monitoring of clinical and blood gas parameters.
Conclusions
In this observational study of patients receiving NIV in our institution, based on the established protocols, NIV was found to be a useful modality in management of hypercapnic respiratory failure of various etiologies; however, it should be judiciously used in patients with hypoxemic respiratory failure. The disease severity at admission, occurrence of new organ dysfunction, hypoxemic ARF, and delay in improvement in PaO2/FIO2 at 1 hour from baseline were independent predictors of poor outcome with the use of NIV.
Footnotes
- Correspondence: Ritesh Agarwal MD DM, Department of Pulmonary Medicine, Postgraduate Institute of Medical Education and Research, Sector 12, Chandigarh 160012, India. E-mail: riteshpgi{at}gmail.com; agarwal.ritesh{at}pgimer.edu.in.
The authors have disclosed no conflicts of interest.
- Copyright © 2012 by Daedalus Enterprises Inc.
References
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.
- 39.↵
- 40.↵
- 41.↵
- 42.
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.
- 64.
- 65.↵