Abstract
Pulmonary zygomycosis is an uncommon infection that occurs mostly in immunocompromised patients. We report the case of a 75-year-old man with myelodysplastic syndrome, treated with lenalidomide for 3 months, who developed respiratory failure and a rapidly progressive left upper lobe consolidation. An extensive workup was unrevealing of the etiology, and the patient expired. A full autopsy was declined, but an in situ post-mortem transbronchial lung biopsy revealed pulmonary zygomycosis. This unique case illustrates the potential risks of lenalidomide therapy in patients with myelodysplastic syndrome and the difficulties in diagnosing pulmonary zygomycosis. To our knowledge this is the first report of a diagnostic in situ post-mortem transbronchial lung biopsy.
Introduction
Myelodysplastic syndrome (MDS) is characterized by dysplasia of myeloid cells, which can manifest as anemia, neutropenia, and/or thrombocytopenia. Treatment of MDS can include lenalidomide, which is a potent analog of thalidomide, which modulates both the cytokine and tumor cell microenvironment.1 This modulation can result in neutropenia and increase the risk of infection.
Zygomycosis refers to an infection caused by fungi including the Rhizopus and Mucor species. Pulmonary zygomycosis is an uncommon infection that occurs mostly in immunocompromised patients, including those with diabetes mellitus, hematologic malignancies, immunosuppressive medications, and neutropenia.2 Pulmonary zygomycosis has a mortality rate of 76%,3 due in part to difficulties in early diagnosis and resistance to commonly used empiric antifungal therapies.
Case Reports
A 75-year-old male with a history of Wegener granulomatosis in remission, end-stage renal disease on dialysis, and MDS refractory anemia with excess blasts-II, treated with 3 months of lenalidomide, presented with fevers and cough productive of brown sputum. Forty days prior to admission, the patient's absolute neutrophil count nadir was 1,060 cells/μL and hemoglobin A1c was 6.1%. Laboratory studies on presentation included white-blood-cell count of 3.1 × 1,000 cells/μL, absolute neutrophil count 2,387 cells/μL, hemoglobin 8.7 g/dL, and platelet count 30 × 1,000 cells/μL. Chest computed tomogram showed multiple pulmonary consolidations that were new or increased from prior imaging, and a new left upper lobe consolidation surrounded by ground-glass opacities. Lenalidomide was discontinued, and the patient was started empirically on vancomycin, cefepime, and voriconazole.
The patient developed hemoptysis and increasing consolidation on imaging, despite broad-spectrum antibiotics. Bronchoscopy with bronchoalveolar lavage (BAL) was done. From the BAL, Gram stain was without white blood cells or microorganisms, bacterial cultures grew commensal respiratory flora, and fungal and mycobacterial cultures had no growth. Assay for (1,3)-B-D-glucan and aspergillus galactomannan antigen were negative. Serum anti-neutrophil cytoplasmic antibody was negative.
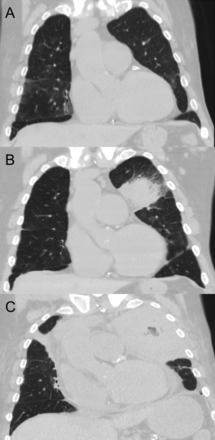
Chest x-ray showed progression of the left upper lobe consolidation. The patient developed hypoxemic respiratory failure and was intubated. His antibiotic regimen was changed to levofloxacin, vancomycin, piperacillin-tazobactam, and voriconazole. Due to concerns for anti-neutrophil cytoplasmic antibody-negative Wegener granulomatosis exacerbation, methylprednisolone and therapeutic plasma exchange were started. Despite these interventions, a repeat chest computed tomogram on hospital day 10 showed interval worsening of left upper lobe opacity with mass effect on the mediastinum. Due to progression of the opacity, voriconazole was discontinued and liposomal amphotericin B was started for broader antifungal coverage (Figure 1).
A: Baseline chest computed tomogram (CT) 2 months prior to presentation. B: Chest CT at presentation, with dominant left upper lobe opacity. C: Chest CT at hospital day 11, with progression of left upper lobe opacity and mass effect on mediastinum.
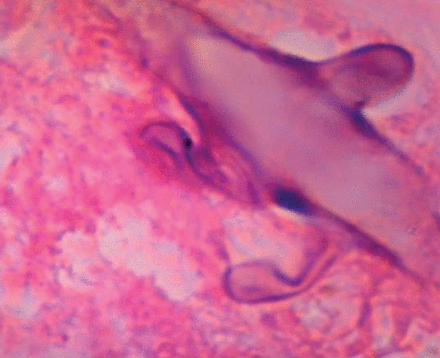
His respiratory status continued to decline, and the decision was made to withdraw care on hospital day 12. A full autopsy was declined, but post-mortem transbronchial biopsy of the left upper lobe was performed and revealed zygomycetes (Figure 2).
Gram-stain (100×) showing broad, pauci-septate, ribbon-like hyphae with wide-angle branching characteristic of zygomycosis.
Discussion
This is a case of pulmonary zygomycosis in a non-neutropenic patient with MDS. Pulmonary zygomycosis is an uncommon infection that occurs most often, but not exclusively, in immunocompromised patients. This patient did not have diabetes mellitus and was not neutropenic on presentation, with an absolute neutrophil count of 2,387 cells/μL. MDS has been associated with fungal infections, although most often in patients who have received induction chemotherapy or have prolonged or severe neutropenia.4,5 He was started on lenalidomide 3 months prior to presentation, which is a tumor necrosis factor-alpha inhibitor 50,000 times more potent than thalidomide.6 This anti-tumor necrosis factor-alpha effect may increase the risk for fungal infections, even in non-neutropenic patients.7 This potential increased risk for fungal infections in non-neutropenic patients on lenalidomide is important for oncology and pulmonary physicians to consider when initiating therapy and managing subsequent fevers and infections.
This case also illustrates the difficulty in diagnosing pulmonary zygomycosis. This patient had a BAL with no evidence of fungal infection and negative assays for (1,3)-B-D-glucan and aspergillus galactomannan antigen. However, the role of BAL in the diagnosis of pulmonary zygomycosis remains incompletely studied. A case series by Glazer et al in 2000 examined 5 patients diagnosed with pulmonary mucormycosis and found microscopy of BAL was positive in 3 of 5 patients, and BAL fungal culture was positive in only 1 of 5 patients.8 (1,3)-B-D-glucan assay and aspergillus galactomannan antigen are negative in zygomycosis because of limited glucan and galactomannan in their cell wall.9 Often a tissue sample is required to make the diagnosis. Commonly employed techniques of obtaining tissue include transbronchial biopsy, imaging guided biopsy, or open surgical biopsy. Unfortunately, this patient was at high risk for complications and thus a biopsy was deferred. Given the limited sensitivity of BAL, lack of cell-wall markers, and the frequent need for a biopsy, the diagnosis of pulmonary zygomycosis is difficult and often delayed. This delay likely contributes to the 76% mortality rate of pulmonary zygomycosis,3 because zygomycosis is not susceptible to voriconazole and the echindocandins, which are often used for empiric antifungal coverage. In this patient the treatment of choice, liposomal amphotericin B (with posaconazole emerging as a new option), was empirically instituted too late to alter the disease course.10
Another important aspect in the treatment of pulmonary zygomycosis is surgical management. A combination of surgical resection and antifungal management is associated with a mortality benefit in the treatment of pulmonary zygomycosis over medical management alone.11,12 In this patient, surgery was not pursued because the surgical risk was high and the diagnosis was not known until post-mortem.
Finally, to the best of our knowledge, this is the first time post-mortem transbronchial lung biopsy has been used without a concurrent autopsy. In this case the family declined full autopsy, which would have been helpful in determining the etiology of the consolidation as well as determining if dissemination had occurred. The family did agree to a less invasive transbronchial lung biopsy, which revealed zygomycetes. This is highly suggestive of pulmonary zygomycosis; however, we cannot entirely rule out colonization. The technique of post-mortem transbronchial biopsy may be a useful alternative in select patients when autopsy is declined.
Acknowledgments
The authors wish to thank Dr James E Kirby for his assistance in obtaining pathology images.
Footnotes
- Correspondence: Jesse Theisen-Toupal MD, Division of Internal Medicine, Department of Medicine, Beth Israel Deaconess Medical Center, 330 Brookline Avenue, Boston MA 02215. E-mail: jtoupal{at}bidmc.harvard.edu.
The authors have disclosed no conflicts of interest.
- Copyright © 2012 by Daedalus Enterprises Inc.