Abstract
Oxygen therapy is extensively used in premature infants and adults with respiratory insufficiency. In the premature infant the goal during manual control of the FIO2 is to maintain adequate oxygenation and to minimize the exposure to hypoxemia, hyperoxemia, and oxygen. However, this is frequently not achieved during routine care, which increases the risks of associated side effects affecting the eye, lungs, and central nervous system. In the adult the primary goal is to avoid hypoxemia, but conventional methods of oxygen supplementation may fall short during periods of increased demand. On the other hand, there are growing concerns related to unnecessarily high FIO2 levels that increase the exposure to hyperoxemia and excessive oxygen use in settings where resources are limited. Systems for automated closed loop control of FIO2 have been developed for use in neonates and adults. This paper will give an overview of the rationale for the development of these systems, present the evidence, and discuss important advantages and limitations.
Introduction
Oxygen therapy is extensively used in neonatal and adult intensive care, during pre-hospital transport, and at home in patients with chronic respiratory insufficiency. In the neonatal population, oxygen supplementation is essential for the survival of newborns in respiratory failure, and the need for supplemental oxygen is particularly frequent and prolonged among premature infants. In this population, the need for supplemental oxygen can extend from the initial stages of respiratory failure and convalescence to a more chronic dependence. In premature infants, excessive and prolonged oxygen supplementation has been associated with systemic oxidative damage and long-term complications affecting the eye, lungs, and central nervous system.1–4 On the other hand, insufficient oxygenation has also been associated with detrimental effects on the brain, pulmonary vasculature, patency of the ductus arteriosus, and other organs and tissues.5–11 Of greater concern is the possible influence of hypoxemia on mortality, as indicated years ago by the increase in mortality after curtailment of the use of oxygen,12–14 and more recently by trials showing increased mortality when targeting lower oxygenation to prevent the occurrence of retinopathy of prematurity and bronchopulmonary dysplasia.15,16
In order to reduce the risk of complications, the goal during manual control of the FIO2 in preterm infants is to maintain adequate oxygenation and minimize the exposure to hypoxemia, hyperoxemia, and oxygen. For this, arterial oxygen saturation is most commonly monitored by pulse oximetry (SpO2) and FIO2 is titrated to maintain SpO2 within a clinically intended range. However, it has been shown that preterm infants spend approximately half of the time within the clinically intended range of SpO2, and more than one third of the time above this range during routine neonatal intensive care.17 Maintenance of SpO2 within the intended range is affected by the infant's respiratory instability, leading to frequent fluctuations in SpO2, to limitations in staff availability to respond to SpO2, and to tolerance of high SpO2 levels by the clinical staff.18,19 In order to overcome these limitations, improve maintenance of target range of SpO2, and minimize hyperoxemia, hypoxemia, and exposure to oxygen, systems of automated control of FIO2 have been developed for use in this population.
Oxygen supplementation is widely used in adult intensive care patients and during pre-hospital transport. In these patients the primary goal of oxygen therapy is the avoidance of hypoxemia, and the use of oxygen is quite liberal, because the risks of side effects associated with hyperoxemia or exposure to high FIO2 are generally considered to be small. There are, however, emerging concerns, including the association between both hyperoxemia and hypoxemia, with increased risk of death post cardiac arrest20 and increased death or worse outcome in severe head trauma.21 Clinicians suggest potential benefits of FIO2 titration in lieu of a continuously high FIO2, independent of the arterial oxygen levels,22,23 and potential risks due to masking of the onset of worsening in lung function by hyperoxemia.24 Also, optimal use of oxygen during air or ground pre-hospital transport is important because oxygen resources may be limited.25
Prolonged oxygen supplementation is common in adult patients with COPD. In these patients supplemental oxygen is adjusted to maintain adequate oxygenation, but conventional oxygen delivery methods may fall short during periods of increased demand, when patient activity increases. Consumption of oxygen resources is a concern in these patients because oxygen delivery methods are designed to facilitate patient mobility.20
Based on these concerns, and with the goal of balancing the maintenance of adequate oxygenation with a rational use of resources, systems of automated closed loop control of FIO2 for adult patients have recently been developed.
Systems of Closed Loop FIO2 Control
Closed loop FIO2 control systems available for clinical or experimental use generally consist of an oxygenation monitoring device (eg, pulse oximeter), gas delivery device (eg, ventilator or cannula), and an algorithm that determines the timing and magnitude of the FIO2 adjustments.
Oxygenation Monitoring
Over the years, closed loop FIO2 systems for neonates and adults have utilized various methods, including indwelling PaO2 electrodes, transcutaneous PO2 electrodes (PtcO2), or arterial oxygen saturation by pulse oximetry (SpO2). A key requirement for the monitoring method used in a closed loop FIO2 system is the ability to provide information on the oxygenation status on a continuous basis, throughout the entire duration of the oxygen supplementation.
The use of indwelling PaO2 electrodes for closed loop FIO2 control is limited by the need of an invasive catheter in place. In neonates, invasive catheters are used during the acute phase of respiratory failure, but their use declines beyond this phase. PtcO2 electrodes require frequent calibration, and their accuracy depends on electrode temperature and perfusion. Hence, frequent changes in application site are required to avoid thermal skin injury in the preterm neonate. More recently, SpO2 has become the preferred method for continuous oxygenation monitoring in neonatal and adult intensive care. This is primarily due to its noninvasiveness and a simple, calibration-free setup. The continuous availability of SpO2 makes this method suitable for use in closed loop FIO2 control systems.
Control Algorithms and Oxygen Delivery Devices
Systems for closed loop FIO2 control are based on algorithms that determine the timing, magnitude, and frequency of each adjustment to keep SpO2 at the target level or range set by the clinician. The measured SpO2 is continuously or intermittently compared to the set target, and the FIO2 adjustments made by the algorithm are inversely related to their difference. These algorithms may also incorporate adjustments in FIO2 based on trend data or the duration of the out of range fluctuation in SpO2.
The timing, magnitude, and frequency of adjustment determine the type of response to the changes in SpO2. These characteristics in the algorithms are tuned to meet the desired objectives for which the system is developed, and therefore define the applicability to different patient groups and the types of fluctuations it is capable of responding to. For instance, a system designed to respond only to slow changing fluctuations in SpO2 is unlikely to provide a timely response during acute and rapid changes in SpO2.
The response to an episode of hypoxemia must be adequate to assist in the recovery, but without producing a rebound hyperoxemia or overshoot. For this, optimization of the timing of the automatic increase in FIO2 when hypoxemia is detected, and the timing when FIO2 is brought back to baseline after the hypoxemia resolves are both important. A delayed increase in FIO2 when SpO2 is trending upwards is likely to result in overshoot into hyperoxemia, while a delayed return of FIO2 to baseline can prolong its duration.
Beat to beat SpO2 values are generally averaged over a running window before they are read by the closed loop algorithm. Long averaging windows attenuate the effects of short fluctuations in SpO2, but they also influence the response of the system. While on one hand, avoiding a response to short lived fluctuations is desirable, delayed or out of phase responses due to long averaging windows that do not reflect the true rate of change in SpO2 may also be counterproductive. A delayed increase in FIO2 because of late detection may prolong hypoxemia, while a delayed return of FIO2 to baseline after hypoxemia has actually resolved may lead to hyperoxemia.
Gas delivery devices used in closed loop FIO2 systems include mechanical ventilators, nasal CPAP devices, nasal cannula devices, and head boxes (also known as hoods). In ventilators and head boxes the air-oxygen mixture is done with blenders that should be sufficiently precise and sturdy to withstand the greater number of small changes required by automatic systems. In nasal cannula systems, the mixing of gases can be achieved by air-oxygen blenders or by modulating the infusion of oxygen to the inspired gas supply. Depending on the intended goal and type of patient, closed loop FIO2 systems may require certain flow rates to produce the changes in the inspired gas within a pre-defined time to achieve a desired effect. Delays in gas mixing may produce out of phase responses between the measured changes in SpO2 and the actual change in FIO2 received by the patient.
The design of closed loop FIO2 systems aimed at responding to hypoxemia events encounters a dilemma when determining the most adequate response when the measured hypoxemia by SpO2 is associated with motion artifact or poor signal quality. This involves the risk of unnecessary oxygen exposure if the measured hypoxemia is due to artifact, versus the risk of exposure to hypoxemia if this is a true hypoxemia event that is accompanied or triggered by patient activity. Avoidance of unnecessary oxygen exposure is desirable, but lack of response during a true event of hypoxemia may have acute consequences. A safe approach during automated FIO2 control is to respond to hypoxemia even if artifact is suspected and to alert the caregiver of the condition for evaluation.
Periods of missing SpO2 measurements due to extremely low signal quality or “drop-outs” can occur during patient activity, decreased perfusion, or probe disconnection. The criteria utilized by closed loop FIO2 systems to adopt a fall back state and the FIO2 to be set are important. The fall back FIO2 can be set by the clinician or by the algorithm based on recently supplied FIO2 levels. The selection of the fall back FIO2 should consider if hypoxemia can be temporally associated with patient activity and therefore go undetected during “drop outs” caused by motion artifact.
Effects of Closed Loop FIO2 on Oxygenation, Oxygen Exposure, and Resource Utilization
In the Neonate
Multiple studies have compared the efficacy of closed loop FIO2 systems to manual adjustments in maintaining oxygenation targets.26–35 These studies have utilized different modes of manual FIO2 adjustment for comparison, including the conventional routine care and more ideal modes consisting of a caregiver fully dedicated to the titration of FIO2. In these studies, automated FIO2 control systems were consistently more effective than manual FIO2 control during routine care in maintaining the oxygenation targets and were similar or better than a fully dedicated caregiver (Table 1).
Maintenance of the Oxygenation Target in Neonates
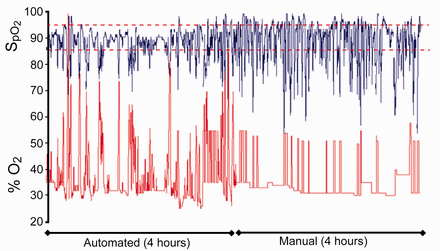
It is important to note in these studies that closed loop FIO2 systems were evaluated in different groups of infants: those receiving invasive mechanical ventilation, nasal CPAP, or oxygen by hood. The studied populations also varied in regards to the frequency and severity of the fluctuations in oxygenation. Infants with frequent episodes of hypoxemia are the most challenging group of infants for the clinical staff and an automated system. An example is shown in Figure 1, where recordings of SpO2 and FIO2 illustrate the response of the automatic system and the substantial work load involved in manual titration of FIO2. The consistency in the findings of improved maintenance of the oxygenation target across these different patient groups provides proof of principle and attests to the feasibility of this approach in the care of the general preterm infant population.
Recordings of SpO2 and FIO2 during 4 hours of automatic and manual adjustment of FIO2 from an infant who presented with frequent episodes of hypoxemia illustrate the response of the automatic system to maintain SpO2 within the target range (dashed lines) and the effort involved in manual titration of FIO2.
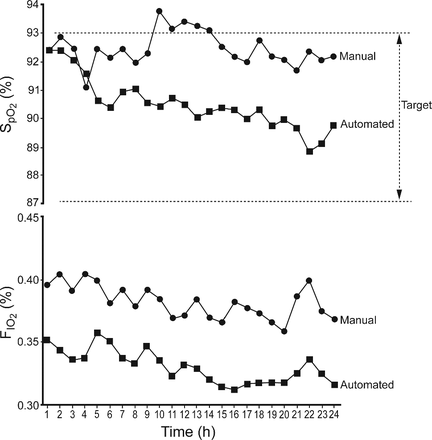
The improved maintenance of the oxygenation targets was largely the result of a reduction in the time spent in hyperoxemia (Table 2), which was more evident in studies comparing closed loop FIO2 to manual adjustments during routine care.34,35 These findings also reflected the tolerance of high SpO2 levels by the clinical staff during routine care. In contrast, in studies comparing closed loop FIO2 to a fully dedicated nurse who was attentive to avoid hyperoxemia, the improved maintenance of the oxygenation targets was the result of a combined reduction in the time spent with SpO2 above and below the target range.32 Also shown in Table 2 is the reduction in FIO2 achieved by closed loop FIO2 systems. A more effective and consistent reduction in FIO2 may be beneficial in premature infants at risk of oxidative lung injury. Figure 2 illustrates with 24 hour data from Claure et al35 how the clinical staff maintained SpO2 near the upper limit of the target range, while the automatic system shifted SpO2 toward the center of the target range over time. This was accompanied by a consistent reduction of FIO2 soon after the infants were switched to the automatic system.
Oxygenation Above the Oxygenation Target and FIO2 in Neonates
Hourly median SpO2 and FIO2 values from 24 hour data from Claure et al35 illustrate maintenance of SpO2 near the upper limit of the target range by the clinical staff, while the automatic system shifted SpO2 toward the center of the range over time. FIO2 was lowered soon after the infants were switched to the automatic system and remained consistently below the levels provided by the clinical staff.
The proportion of time infants spent with oxygenation levels below the target in the different studies was not reduced by closed loop FIO2 as consistently as the reduction in hyperoxemia (Table 3). Although these observations seem at first counterintuitive, they underline the fact that closed loop systems can respond to the occurrence of spells of decreased SpO2, but they are ineffective in their prevention. Also, because hypoxemia spells in preterm infants are largely triggered by changes in ventilation and lung volume, the automatic increase in FIO2 can attenuate the severity or duration of the episode, but it is unlikely to produce an immediate resolution of the episode of hypoxemia.35 In studies focused on preterm infants who present with frequent hypoxemia episodes, there was an increased number of mild episodes during closed loop FIO2 control, in comparison to manual adjustments during routine care. This difference was largely related to the maintenance of relatively high basal SpO2 levels by the staff trying to prevent the spells of hypoxemia. This preventive strategy was only partially effective in preventing hypoxemia episodes, but increased the exposure to oxygen. In contrast, closed loop FIO2 control was more effective in reducing the number of the more severe and prolonged episodes of hypoxemia.
Oxygenation Below the Oxygenation Target in Neonates
The improved maintenance of SpO2 targets by automatic FIO2 control was achieved in spite of a considerable effort of the clinical staff. FIO2 was adjusted by the staff an average 8.5 times per hour during day time,34 and 113 times during a 24 hour period.35 These findings suggest potential reductions in work load that may also enable a more effective use of the caregiver's effort toward the improvement of other areas of care.
In the Adult
Studies have compared multiple systems for automated control of FIO2 to the conventional modes of oxygen administration in mechanically ventilated trauma intensive care patients, and in COPD patients who receive long-term oxygen therapy during exercise and normal activity, as well as during induced hypoxemia in adult volunteers.36–39 In these studies (Table 4) closed loop FIO2 systems set to maintain SpO2 between 90% and 96% have consistently improved the maintenance of such range, compared to conventional care consisting of either a manually titrated or a constant oxygen supply. More importantly, these studies documented that automated closed loop control of FIO2 was more effective in reducing hypoxemia, which is the primary concern in these patients (Table 5). These findings suggest that the eventual benefits of closed loop FIO2 may be related not only to less hypoxemia, but also to facilitating increased activity levels in chronic patients who, without manual titration of the supplemental oxygen, would become hypoxemic.
Maintenance of the Oxygenation Target in Adults
Oxygenation Below Target in Adults
These data confirmed that high SpO2 levels are common and prolonged, which involves considerable oxygen utilization (Table 6). The time spent with high SpO2 was consistently reduced by closed loop FIO2, compared to the conventional care, without leading to detrimental effects. These findings not only document the efficacy of closed loop FIO2 in these populations, but also suggest that hyperoxemia did not confer a tangible advantage in those patients. The observed oxygen resource savings may be appealing for applications such as transport or remote facilities where closed loop FIO2 systems may lead to a more efficient oxygen utilization. Also important is the potential reduction in the effort required for manual adjustment of the inspired oxygen and better utilization of the staff.
Oxygenation Above Target and Consumption of Oxygen Resources in Adults
New systems that combine automatic adjustments of ventilator parameters, such as minute ventilation and peak inspiratory pressure, in parallel to automatic adjustment of FIO2 and PEEP, have recently become available.40,41 Reports on the use of these systems in adult ventilated patients with acute lung injury and post-cardiac surgery indicate better maintenance of the target ranges of oxygenation and other physiologic targets, less hyperoxemia, and reductions in FIO2. These more sophisticated systems also resulted in fewer manual interventions from the clinical team to adjust the ventilator settings.
Important Considerations and Possible Disadvantages
In both neonatal and adult populations the most important aspect to be considered by the clinician when determining the use of closed loop FIO2 is the target range of SpO2. In both populations the optimal range of SpO2 has not been clearly defined, and most of the currently recommended ranges may not truly represent the actual levels of SpO2. Although the primary benefit of closed loop systems may be given by the avoidance of the extreme ranges of SpO2, there may be important physiologic and clinical effects of specific target ranges that have not yet been determined. A more consistent maintenance of specific target ranges by automated systems may uncover effects that have not been previously documented. Hence, cautious selection of the target range is warranted, because any adverse or beneficial effect may not be attributable to the automated approach but to the better maintenance of such range.
Closed loop FIO2 systems are dependent on the reliability and accuracy of pulse oximetry. Although closed loop algorithms can validate the reliability of SpO2 and enter into fall back state, the clinician determining the use of closed loop FIO2 in an individual patient should apply the same or even stricter criteria as the one used routinely to monitor and assure the reliability of pulse oximetry in every patient.
Automated control of FIO2 provides a single parameter response to hypoxemia, whereas the caregiver response, if appropriate, would determine the mechanism producing hypoxemia, followed by the appropriate intervention. For instance, increased FIO2 may not be the most adequate response when the hypoxemia is due to hypoventilation. However, it should be noted that clinical staff frequently respond first by increasing FIO2 to attenuate the hypoxemia and then investigate the cause. The likely earlier automatic response could further attenuate the hypoxemia until the corrective intervention is done. Ventilation monitoring, assessment, and identification of the mechanism producing hypoxemia that should be part of the standard care must also be part of the care during automated control of FIO2. This is important, because one possible drawback of the use of automated FIO2 control systems is that they could inadvertently reduce the attentiveness of the caregiver and delay recognition of changes in respiratory function. To minimize this risk of masking an ongoing deterioration in respiratory function by the automatic increase in FIO2, closed loop FIO2 systems should warn of persistent increases in FIO2, even if SpO2 is within the target range in order to prompt assessment by the clinician. The occurrence of these unwanted effects has not yet been shown, but, nonetheless, adequate training and careful use of these systems are warranted.
Summary
In premature infants, important detrimental effects have been associated with insufficient oxygenation as well as hyperoxemia and excessive exposure to high FIO2 levels. These risks are increased because of the infant's respiratory instability and the limitations of conventional care in maintaining the target ranges of oxygenation. Available data indicate the feasibility of automated closed loop control of FIO2 in this population and suggest potential benefits by achieving a better oxygenation control while limiting oxygen exposure. The long-term effects of this approach in these population still need to be assessed.
In the adult, oxygen supplementation is primarily used to avoid hypoxemia, with generally little concern about hyperoxemia. This, however, is being increasingly challenged by concerns of the possible noxious effects of hyperoxemia. These concerns, along with the possible benefits of better oxygen utilization and reduced staff effort, have stimulated the development of automated FIO2 control systems that have shown promising results. These include better maintenance of the oxygenation targets, less hypoxemia and hyperoxemia, and reduced oxygen use. The long-term benefits of this approach in the adult population also need to be further explored.
Studies of automated control of FIO2 have also documented the potential for substantial reductions in work load. Therefore, the possibility exists that use of these systems may indirectly improve care by shifting the staff effort to other patient care functions.
Discussion
Kallet:
One thing just occurred to me, at least in terms of the adult. As a clinician, if it's busy in the unit and I have someone who may be on 60% or 70% O2 with a stable SpO2, I might say to myself I really don't have time to wean them, because if I do and they become unstable, I'm not sure I can get back to them quickly enough. One of the potential implications for adults is that, with automatic titration, work load and acuity will have less of an impact if you have an automatic titration and a way to monitor how stable they are at the lower FIO2. It might actually facilitate weaning.
The other thing is that more severely ill patients with ARDS may have borderline PaO2, but if you titrate them down in 5% steps, they actually maintain saturation. So I think there's a psychological component to this. The clinician may be reluctant to wean the FIO2 in a tenuous patient they're not confident will do OK with the change. There might be something very nice about an automatically titrated system that may affect outcomes.
Claure:
Very important point. I agree. There are additional potential benefits in intensive care in general and across different patient groups. It might have indirect benefits because of the more consistent approach, not only in responding to hypoxemia, but in weaning FIO2 that may exceed what is necessary for a given patient.
MacIntyre:
I was thinking of this from the adult perspective as well, and I can see situations where if you have hypoxemia, and your normal response is to adjust the FIO2, this system could be very useful. A classic example would be in the home, where you increase your activities or exercise and you have an FIO2 response situation. I'm having a lot of difficulty imagining this in the ICU, because there are many other manipulations you may want. For instance, you may want to adjust PEEP or assess suctioning needs or minute ventilation or hemodynamics, and having a system that's going to be adjusting the FIO2 to keep the SpO2 alarm from going off just seems a little simplistic for me right now. Are there ways of using this kind of feedback system and incorporating a PEEP/FIO2 algorithm so it's not just FIO2 adjustments?
Claure:
This is a very important issue and relevant not only in adults. A change in oxygen may not be the most appropriate response. The patient may benefit from addressing the root cause of the hypoxemia. Unfortunately, it does not always happen, and the first response is often to increase the O2, which is followed by assessment. Many times ventilation parameters are not monitored or assessed.
There are systems that adjust PEEP and FIO2 simultaneously in adults and pediatric patients.1,2 Systems developed for neonates can increase the mandatory rate of the ventilator in the event of hypoxemia.3 There is a risk that closed-loop FIO2 may reduce attentiveness, but there are methods the staff can use to monitor ventilation and tidal volume. Alarm systems can alert the user when saturation is adequate but the patient is requiring more O2 than before. Obviously, nothing will replace the proper assessment and interventions the experienced clinician can do at the bedside. We see closed-loop FIO2 as a tool to assist in the repetitive task of increasing or decreasing oxygen.
Kevin Ward:
What is the pathway through the FDA for this?
Claure:
My involvement is primarily as an investigator. Our center is one of the centers participating in gathering the data to support the application to FDA. The process may be long, but seeking approval in the United States is necessary.4,5 In reference to the pathway, CareFusion, the device manufacturer, will be able to answer your question directly.
Owens:
The neonatal data you showed reminds me of central sleep apnea in an adult. You are focused on O2 saturation, but the drops in O2 saturation are likely driven by changes in ventilation. Have you looked at incorporating end-tidal CO2 measurements? You mentioned using adaptive servo ventilation and trying to increase ventilation rather than just respond with more O2 during apneas. Is that something you've done? In the adults some people use dead space or bleed-in CO2 to keep ventilation stable. Have you looked at closed-loop ventilation versus closed-loop O2?
Claure:
Yes, we have worked with volume-targeted ventilation, where the ventilator increases the pressure trying to maintain tidal volume within an adequate range. It is partly effective. It doesn't prevent the occurrence of the spells, but it does attenuate the resulting hypoxemia. We have tested targeted minute ventilation (also called mandatory minute ventilation), where the ventilator's mandatory rate increases in the event of hypoventilation, and it is also effective. We also developed and tested a system that increases both the rate and the pressure to maintain both tidal volume and minute volume, and this combined mode is quite effective, but it is still experimental.
Perhaps in the future there will be 2 systems running in parallel: one for closed-loop ventilation and the other for closed-loop oxygen saturation. When an infant is not intubated, things become more challenging, because there is less certainty of the efficacy of a backup ventilator mode. Fortunately, non-intubated infants usually have fewer and less severe fluctuations.
Treggiari:
I have a comment regarding ventilation in adults. I appreciate the value of closed-loop in down-titration of FIO2, but not necessarily for up-titration. Indeed, closed-loop could expedite weaning the FIO2, as sometimes the patient has improved arterial blood gases but it takes several hours before ventilator changes are made. Based on my experience, I think an automatic mechanism would help expedite down-titration, but I would be more concerned with up-titration because, as you mentioned, we need to evaluate the cause of the desaturation. I think it's probably good to differentiate the processes of FIO2 weaning and FIO2 up-titration.
Claure:
I agree. Closed-loop systems are quite effective in weaning. There is more control over oxygenation when FIO2 is being decreased. In contrast, when FIO2 is being increased, there is less control because the problem is usually related to ventilation. Our concern is that during routine care we often see FIO2 increased in what was supposed to be a transient response but actually becomes a prolonged FIO2 increase. The concern is rebound hyperoxemia, which may have serious consequences in premature infants.
Kallet:
In response to that comment and to Neil's [MacIntyre] comments, what might take care of that is a high-baseline alarm. So if you're performing routine care that results in transient desaturation requiring a few minutes to recover, then having a high-baseline alarm that's too vigilant will cause the whole idea to breakdown as a practical tool. If someone goes from 50% O2 to a higher stable baseline of 70% O2 for 20 minutes, then having a secondary alarm that tells you, “This is a real change in condition,” would take care of that. The down side of that is more complexity in the alarms, requiring bedside clinicians to be more sophisticated in handling them. But I think they're on the right track in building up high and low baseline alarm levels. Having independent alarm delay times you could set would take care of a lot of those problems. But it definitely would make things more complicated.
McCoy:
I think the future is auto-adjusting for home care, which takes care of a lot of the problems in home O2 therapy. One of the biggest problems is that patients are told, “Don't touch the dial, because you'll kill yourself,” and if the device allows them to oxygenate without the fear of them turning the O2 up or down, it would be fantastic. I'm hoping the technology will evolve.
Recent articles6,7 on auto-adjusting home application said that the future is in that direction. The comment in the one from Italy was that it over-compensated. One of the things respiratory therapists do in the home is go one setting at a time, and one device went 3 settings in one go and overshot. I've tried to chase patients before where they're desaturating, but I can't catch up because they keep on desaturating, and a slow increase doesn't catch them. So this device is smart enough to start high and then comes back down, which I think is a good approach. I'm looking forward to these devices being commercialized.
Branson:
Neil, I'll answer a couple of your comments. One is that Hamilton has Intellivent, which is ASV [adaptive support ventilation] with a combined FIO2/PEEP controller.8 It uses the high-FIO2/PEEP algorithm from the ARDS Network ARMA trial9 and then the high-PEEP/FIO2 table from the ALVEOLI trial,10 so the FIO2 goes up first, fastest, and then, once that's adjusted, the PEEP starts to be titrated, and then it gets reduced in the opposite direction. It has only very preliminary evidence, from Europe, not the United States.
When we built our system, we were funded by the military, so there are different goals. First, who's caring for the patient? The military medic is like an EMT [emergency medical technician], so understanding how to use a ventilator is kind of beyond their scope. And in the military there's often no access to the patient in the back of the helicopter or other vehicle. Our system is meant to conserve O2 by giving the lowest possible FIO2, and because head injury is the signature injury of the present war, to prevent hypoxemia and secondary brain injury. If SpO2 goes below 88%, it increases the FIO2 rapidly to 100%, and then returns it slowly.
I'm not sure that system would be helpful in a regular ICU, but I have been impressed by the papers by the Dutch group11 and others,12 and a paper in Respiratory Care from the Mayo Clinic demonstrating time after time, especially surgical and neurosurgical patients being on FIO2 of 40 to 50% with PaO2 of 150 mm Hg and absolutely no intervention, not only for hours but for days.13 And that might need to be addressed.
Nelson, what about all this stuff that's come out recently about the target? There's the paper in which14 a lower target was associated with worse outcomes at 36 weeks, and that more hypoxemic events are associated with an increase in ROP [retinopathy of prematurity]. Is it going to be that the machine comes out with a target, or do you think it's important for the clinician to set the target for each individual patient?
Claure:
I think the clinician has to be the one responsible for setting the target. For many years avoidance of hyperoxemia was the goal and oxygen saturation targets were being lowered. Saturation targets under 92% or 90% were being used with the goal of staying away from very high saturation. Randomized trials were conducted testing the hypothesis that targeting below 90% would decrease retinopathy and improve respiratory outcome. In one of these trials, respiratory outcome was improved and the incidence of retinopathy was reduced, but mortality increased.14,15 These findings opened a new set of questions. There are lots of new developments, and everybody is awaiting the results of other large trials, which will report data from over 4,000 cases.16 At this point nobody can really recommend what is the optimal target saturation range for preterm infants. I do think avoiding the 2 extremes ranges of oxygen saturation is necessary.
In regards to the fluctuations in saturation, there may be more than subtle effects to these relatively low saturations, especially if the exposure is prolonged. It should be noted that the actual effects of the target range are not necessarily attributable to the closed-loop systems. A closed-loop FIO2 system will target the range set by the clinician, and if the range is not adequate, the results are not necessarily due to the closed-loop system.
Footnotes
- Correspondence: Nelson Claure MSc PhD, Division of Neonatology, Department of Pediatrics, University of Miami Miller School of Medicine, PO Box 016960, R-131, Miami FL 33101. E-mail: nclaure{at}miami.edu.
Dr Claure presented a version of this paper at the 50th Respiratory Care Journal Conference, “Oxygen,” held April 13–14, 2012, in San Francisco, California.
The authors have a patent on a closed loop oxygen system discussed herein. The University of Miami is the assignee for that patent and has a licensing agreement with CareFusion, which provided support for studies of the system.
- Copyright © 2013 by Daedalus Enterprises Inc.