Abstract
BACKGROUND: Aspiration of colonized oropharyngeal secretions is a major factor in the pathogenesis of ventilator-associated pneumonia (VAP). A tapered-cuff endotracheal tube (ETT) has been demonstrated to reduce aspiration around the cuff. Whether these properties are efficacious in reducing VAP is not known.
METHODS: This 2-period, investigator-initiated observational study was designed to assess the efficacy of a tapered-cuff ETT to reduce the VAP rate. All intubated, mechanically ventilated patients over the age of 18 were included. During the baseline period a standard, barrel-shaped-cuff ETT (Mallinckrodt Hi-Lo) was used. All ETTs throughout the hospital were then replaced with a tapered-cuff ETT (TaperGuard). The primary outcome variable was the incidence of VAP per 1,000 ventilator days.
RESULTS: We included 2,849 subjects, encompassing 15,250 ventilator days. The mean ± SD monthly VAP rate was 3.29 ± 1.79/1,000 ventilator days in the standard-cuff group and 2.77 ± 2.00/1,000 ventilator days in the tapered-cuff group (P = .65). While adherence to the VAP prevention bundle was high throughout the study, bundle adherence was significantly higher during the standard-cuff period (96.5 ± 2.7%) than in the tapered-cuff period (90.3 ± 3.5%, P = .01).
CONCLUSIONS: In the setting of a VAP rate very near the average of ICUs in the United States, and where there was high adherence to a VAP prevention bundle, the use of a tapered-cuff ETT was not associated with a reduction in the VAP rate.
Introduction
Ventilator-associated pneumonia (VAP) is a common nosocomial infection in the ICU.1 Five to 20 percent of patients who require mechanical ventilation for > 48 hours will develop VAP, and the associated mortality is 15–50%.2,3 ICU stay in patients with VAP is increased by a mean of 6.1 days, and the excess cost can be as high as $40,000 per patient.4,5 These costs are secondary to increased use of antibiotics and longer mechanical ventilation ICU stay. Therefore, VAP prevention can reduce both the cost and morbidity associated with mechanical ventilation.
Aspiration of colonized oropharyngeal secretions is one of the primary factors implicated in the pathogenesis of VAP. VAP prevention efforts have centered on reducing the bacterial load of aspirated secretions, and reducing the amount of secretions aspirated.6 With regard to the former, disinfection of the oropharynx with chlorhexidine, coupled with increased attention to oral care, has been shown to reduce the risk of VAP.7 Similarly, elevation of the head of the bed has been demonstrated to reduce the risk of aspiration of oropharyngeal secretions into the lung in intubated, mechanically ventilated patients and has become a nearly universally applied element to prevent VAP.8,9 Applying each of these interventions as a “bundle” has resulted in marked reduction in the incidence of VAP.10,11 However, despite these measures, VAP remains an important problem, with a reported incidence of approximately 2 cases per 1,000 ventilator days.12
Oropharyngeal secretions pool above the cuff of the endotracheal tube (ETT) and can be aspirated into the trachea despite the presence of the cuff, and may represent an important pathway for colonized secretions to enter the distal airway. Recently, modifications to the design of the ETT cuff have been the focus of interventions to reduce the risk of aspiration around the cuff, and thus reduce the risk of VAP. Employing a tapered cuff, as opposed to the standard barrel-shaped cuff, has been proposed to provide a better tracheal seal and reduce the passage of potentially contaminated secretions around the cuff and into the lung. A recent trial employed methylene blue instilled above the ETT cuff in pigs undergoing abdominal surgery. Significantly less methylene blue staining of mucosa following surgery was observed in pigs intubated with a tapered-cuff ETT, compared with those intubated with a standard barrel-shaped-cuff ETT.13 A tapered-cuff ETT (TaperGuard, Covidien, Mansfield, Massachusetts) has been approved by the FDA, and in vitro and in vivo studies have demonstrated reduced aspiration around the cuff.1 Whether these properties are efficacious in reducing VAP is not known. These specialized ETTs are more expensive than standard ETTs, but represent a “passive” intervention to reduce VAP; they require little to no additional effort on the part of care providers. As a component of institutional efforts to evaluate VAP-prevention measures, we performed a sequential observational study to test the hypothesis that hospital-wide use of a tapered-cuff ETT would result in a significant reduction of the VAP rate (VAPs/1,000 ventilator days).
QUICK LOOK
Current knowledge
Leakage of colonized oropharyngeal secretions around the endotracheal tube cuff is a major factor in the pathogenesis of ventilator-associated pneumonia (VAP). A tapered-cuff endotracheal tube reduces the leakage in a bench model, but the tapered cuff's ability to alter the incidence of VAP is not known.
What this paper contributes to our knowledge
In an ICU with a VAP rate near the average of United States ICUs, and where there was high adherence to a VAP prevention bundle, the tapered-cuff endotracheal tube was not associated with a reduction in the VAP rate.
Methods
Study Design
This 2-period observational study was approved by our institutional review board, which waived the requirement of informed consent. Each study period was 6 months in duration, and included the same calendar months, to minimize the impact of seasonal variation on incidence of VAP. A transition period, during which the hospital supply of ETTs was changed, was not included in the analysis.
Inclusion Criteria
All patients over the age of 18 admitted to any of our adult ICUs and who required endotracheal intubation and mechanical ventilation at any time during their ICU stay were included. These 110 ICU beds encompassed multiple specialties, including medical ICU, surgical ICU, neurointensive care, cardiothoracic ICU, trauma ICU, and coronary care. The VAP prevention bundle and VAP data collection were identical across all ICUs.
Study Procedure
During the baseline 6 month period, July 1, 2010, through December 31, 2010, a standard barrel-shaped-cuff ETT (Mallinckrodt Hi-Lo, Covidien, Mansfield, Massachusetts) was used hospital wide. All ETTs throughout the hospital (including the operating rooms) were then replaced with a tapered-cuff ETT (TaperGuard, Covidien, Mansfield, Massachusetts). This was performed by resource management staff who purchase and stock all supplies at the medical center. Additionally, our county (Forsyth County, North Carolina) emergency medical response units were supplied with the tapered-cuff ETTs, but intubated patients transferred from other institutions were not re-intubated with a tapered-cuff ETT; however, these patients were included in the study. After a hospital-wide audit to ensure removal of the barrel-shaped-cuff ETTs, the second period of the study commenced on July 1, 2011 and extended through December 31, 2011. The tapered-cuff ETT itself is of the same materials and design as the barrel-shaped-cuff ETT: the difference is solely in the cuff design. This ETT does not have an extra lumen to permit removal of supraglottic secretions, and, other than the shape of the cuff when inflated, is identical in appearance to the standard barrel-shaped-cuff ETT. Therefore, no staff education concerning the replacement ETT was felt to be necessary, though it was announced that a new type of ETT was being studied. Respiratory therapists' procedures for caring for ETTs were unchanged throughout the study period. The respiratory therapists checked and documented the position of the ETT and maintained ETT cuff pressure at 20–25 cm H2O twice during each 12 hour shift.
Data Collection and Definition of VAP
Infection Control practitioners trained in data abstraction and reporting were responsible for capturing all instances of VAP during ICU admission. They were not involved in direct patient care, and each suspected case of VAP was reviewed by the hospital epidemiologist, a board certified specialist in infectious diseases. These individuals did not change during the study period. Criteria for the diagnosis of VAP were concordant with National Healthcare Safety Network VAP criteria (PNU2), which requires bacteriologic confirmation in addition to meeting specific clinical criteria, including, for example, new radiographic infiltrates, fever, and leukocytosis.14 However, in our institution, outside the trauma ICU, instead of the bronchoscopic cultures specified in PNU2, we employ quantitatively cultured tracheal aspirate, and we use a threshold of ≥ 105 colony-forming units (CFUs) per mL to confirm a VAP diagnosis. Within the trauma ICU, bronchoscopic and non-bronchoscopic bronchoalveolar lavage cultures were used, with a threshold of 104 CFU/mL to confirm VAP. This was consistent throughout the study.
Personnel from our quality resource center monitored adherence to the institutional VAP prevention bundle and days of mechanical ventilation. These individuals had no role in the provision of clinical care. These data were collected in all ICU patients, Monday through Friday, prospectively, by chart review and bedside inspection. Data on weekends and holidays were collected retrospectively by chart review. The institutional VAP prevention bundle consisted of elevation of the head of bed to 30°, swabbing all oral surfaces (buccal, pharyngeal, gingival, tongue, and tooth surfaces) for 30 seconds with 15 mL 0.12% chlorhexidine gluconate twice daily, brushing teeth twice daily, general oral care every 4 hours, and orogastric in preference to nasogastric tube. VAP bundle adherence for each intubated subject was recorded daily as adherence to the entire bundle (ie, failure to perform a single component was defined as failure to adhere to the bundle for that ventilator day). Subjects in whom there was a documented medical contraindication to a component of the bundle (eg, a nasogastric tube inserted operatively in a subject undergoing esophageal surgery) were not recorded as non-adherent, provided they were adherent to all other bundle components.
The number of intubated subjects and patient days of mechanical ventilation were recorded, and the VAP rate is expressed as VAPs per 1,000 ventilator days. A day of mechanical ventilation was defined as mechanical ventilation at any time on that calendar day.
Role of the Sponsor
This was an investigator initiated study. The sponsor (Covidien, Mansfield, Massachusetts) provided the tapered-cuff ETTs at a discounted price and provided salary support for data collection. The sponsor was not involved in study design, data collection, or data analysis. The sponsor had no access to study data, nor any role in formulating the conclusions for this report, but was allowed to review the manuscript prior to submission for publication.
Statistical Analysis
Data were entered into a secure, password-protected database, and analyzed with statistics software (SPSS 11, SPSS, Chicago, Illinois). Results were analyzed initially using descriptive statistics. Comparisons between groups were done using chi-square test for proportions, and the unpaired Student t test for continuous variables. A 2-tailed P value < .05 was considered statistically significant. From December of 2009 to June 2010, our institutional monthly VAP rate was 5.8 ± 1.47 per 1,000 ventilator days. We estimated that a 6-month trial period would have an 80% power to detect a 50% reduction in the primary outcome of VAPs/1,000 ventilator days, with an alpha of .05.
Results
We included 2,849 subjects, encompassing 15,250 ventilator days. The distribution of ventilator days was 41% medical ICU, 35% surgical and trauma ICU, 11% cardiothoracic ICU, 10% neurological ICU, and 3% other ICUs. The distribution of subjects across the ICUs did not differ between the study periods. There were 24 VAPs while using the barrel-shaped-cuff ETT (standard-cuff group), and 21 VAPs while using the tapered-cuff ETT (tapered-cuff group) (P = .71) (Table). The mean ± SD monthly VAP rate was 3.29 ± 1.79/1,000 ventilator days in the standard-cuff group, and 2.77 ± 2.00/1,000 ventilator days in the tapered-cuff group (P = .65).
Subjects, Ventilator Days, and VAP Data
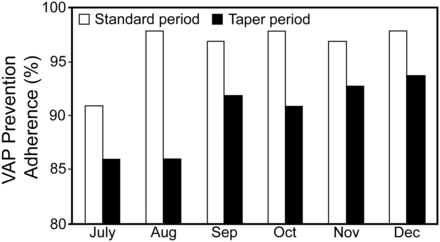
Adherence to the VAP prevention bundle was high throughout the study period. However, VAP bundle adherence was significantly higher during the standard-cuff period (96.5 ± 2.7%) than in the tapered-cuff period (90.3 ± 3.5%, P = .01). The most common reason for VAP bundle non-adherence was failure to perform (or document) every scheduled provision of oral care. This accounted for 76% of all VAP bundle non-adherence. The number of VAPs during each study month, the monthly VAP rate, and VAP bundle adherence are displayed in Figures 1, 2, and 3, respectively. There were no significant differences in the number of mechanically ventilated subjects or the number of ventilated days (patient days of mechanical ventilation) between periods.
Number of ventilator-associated pneumonias (VAPs) during the 2 study periods.
Ventilator-associated pneumonia (VAP) rate (VAPs/1,000 ventilator days) during the 2 study periods.
Adherence to the ventilator-associated pneumonia (VAP) prevention bundle during the 2 study periods.
Discussion
The use of the tapered-cuff ETT did not significantly reduce the VAP rate. Our adherence to VAP-prevention measures was high, but it was significantly higher in the standard-cuff period than in the tapered-cuff period. The reason for this difference is not clear, but the consistency of performance of interventions to improve quality has been reported to wane over time if efforts directed at maintaining performance are not sustained.15 It is possible that reduced adherence to the VAP prevention bundle masked a significant reduction in VAP in the tapered-cuff period. However, adherence rates of 80–90%, as observed in the tapered-cuff period, have been reported to still be associated with a reduced VAP rate.8 Further, an analysis of our VAP rate and VAP bundle adherence from July 2008 through June 2010 found only a weak correlation between VAP bundle adherence and VAP incidence (r2 = 0.11) (unpublished data). Collectively, while this lessens the probability, it does not preclude that the observed difference in VAP bundle adherence obscured a clinically relevant reduction in VAP attributable to the use of a tapered-cuff ETT.
The VAP rate range in the present study, 2.77–3.29/1,000 ventilator days, was much lower than that in other studies reporting successful interventions to reduce VAP, including elevation of the head of the bed, chlorhexidine oral rinsing, silver-coated ETTs, and subglottic secretion removal, in which VAP rates of 10–15/1,000 ventilator days or higher were observed.16–19 Whether significant reductions in VAP consequent to use of a tapered-cuff ETT might be observed in settings with a higher baseline VAP rate cannot be determined from the present study. Importantly, however, the rates reported in the present study closely mirror the median rates reported by the National Healthcare Safety Network for 2010 (range approximately 0–5/1,000 ventilator days, depending upon the type of ICU).12 Several studies have suggested that publicly reported VAP rates may not accurately reflect clinical VAP.20,21 However, a study of patients in our institution indicated that, while specific patients may differ, the rates of pneumonia were similar whether bronchoscopic or National Healthcare Safety Network criteria were used.22
While this study was designed to be powered to detect a 50% reduction in the VAP rate, this was predicated on a historical VAP rate of 5.8 ± 1.47 per 1,000 ventilator days, present in early 2010 during study planning. The observed baseline rate was only 3.29/1,000 ventilator days. With this baseline VAP rate a study duration of 3 years would be required to have an 80% power to detect a 50% reduction. This underscores the difficulty of executing adequately powered studies of VAP prevention in the setting of successful baseline efforts to reduce the VAP rate.
Of note, in contrast to the bronchoscopic quantitative cultures used in the National Healthcare Safety Network PNU2 definition of VAP, quantitative tracheal aspirates with a threshold of 105 CFU/mL were employed to confirm VAP. However, this criterion has been shown to result in a VAP rate virtually identical to the rate found if using a bronchoalveolar lavage quantitative culture threshold of 104 CFU/mL.23,24
Another limitation of this study is that transfer patients were not re-intubated with a tapered-cuff ETT. Patient referral and transfer patterns in our institution were not known to change over the study period, and, while unlikely, an unappreciated increase in these patients with an attendant higher VAP incidence may have masked a decrease in VAP in subjects intubated with the tapered-cuff ETT. While all mechanically ventilated adult patients were included, the limited data elements collected for the study precludes ascertainment of the impact of time-related changes in the demographic characteristics, severity of illness, or comorbidities on the incidence of VAP.
Only VAP occurring in an ICU was captured. However, all patients who were mechanically ventilated were initially cared for in an ICU, and only a small minority are ultimately transferred to a step-down unit for longer-term ventilator management. Only one VAP was observed in the step-down unit during the study period. Thus, comparison of the VAP rates in the standard-cuff and tapered-cuff groups should not be affected by this limitation.
Conclusions
In the setting of a VAP rate very near the average of ICUs in the United States, and where there was high adherence to a VAP prevention bundle, the use of a tapered-cuff ETT was not associated with a significant reduction in the VAP rate.
Footnotes
- Correspondence: David L Bowton MD, Department of Anesthesiology, Wake Forest Baptist Health, Medical Center Boulevard, Winston-Salem NC 27157-1009. E-mail: dbowton{at}wakehealth.edu.
This research was partly supported by Covidien. See Methods for details. Drs Bowton and Hite were partly supported by grants from the National Institutes of Health. Drs Martin and Sherertz have no disclosed no other conflicts of interest.
See the Related Editorial on Page 1704
- Copyright © 2013 by Daedalus Enterprises